Systemic Lidocaine for Perioperative Analgesia: A Literature Review-Juniper Publishers
Authored
by Fabrício Tavares Mendonça
Abstract
Introduction and Background:
Intravenous lidocaine has been increansingly used as pain treatment in
anesthesiology. The aim of this study was to review the scientific
literature on the use of lidocaine for perioperative analgesia, a recent
technique and still under study to demonstrate its clinical
implications.
Methodology: IData were derived from MD Consult e Clinical Key (1998-2014). A total of 32 articles were selected.Results and Discussion: Lidocaine acts by changing the excitatory nerve impulse driving; decreasing visceral pain, central sensitization of pain and the immune response. Intravenous lidocaine with good clinical results were used in the dose of preoperative bolus 1,5 a 2 mg.kg-1followed by continuous infusion 1,5 a 3 mg.kg-1.h-1. Most of clinical trials were in patients undergoing abdominal surgery. It is well-established analgesic, anti-inflammatory and anti-hyperalgesic properties of this local anesthetic; other of its beneficial effects were reduce consumption of volatile anesthetic and opioids, and fasten return of bowel function.
Conclusion: Systemic lidocaine was able to promote great analgesia in surgical procedures. It is a low cost and very convenient alternative on perioperative pain treatment. More clinical controlled studies in different surgical intervention may yield more information about this analgesic approach.
Keywords: Local anesthetics; Pain; Perioperative; Intravenous; Lidocaine
Introduction
Pain is a very common phenomenon on postoperative
period and it is oftenly neglected. Pain control is essential for
surgical patient assistance, as long as persistence of painful stimuli
increase the incidence of complications. Acute pain is related to
nociceptive stimuli produced by tissue damage, which results in a group
of sensitive, cognitive and emotional experiences, generating autonomic
and behavior responses. Acute and persistent painful stimuli may change
nervous system’s plasticity leading to its cronification [1,2].
Coenzyme Q10: A walk through the basic
The goal of pain treatment is blocking the
generation, transmission, perception end sensation of nociceptive
stimuli in different levels of peripheral and central nervous system
[1]. This reduces perioperative morbidity, favors surgical treatment
result, reduces hospital expenses and decreases postoperative chronic
pain risk [3,4].
Nevertheless, many patients submitted to surgical
procedures go through moderate to strong pain on postoperative period,
indicating that despite of the development of new medications and
implementation of different analgesic techniques, postoperative pain
remains misdiagnosed and mistreated [5].
Opioid analgesics are commonly used in clinical
practice for perioperative pain treatment. However, its use is related
to many side effects, as respiratory depression, nausea, vomit,
drowsiness, pruritus, urinary retention, constipation, hyperalgesia and
immunologic function compromise [6]. Therefore, alternative techniques
and medications have been used as substitute of opioid for analgesia,
that is, where well fits the systemic intravenous infusion of lidocaine,
local anesthetic widely used on anesthesiology practice [6-8].
Studies showed that the intraoperative use of
lidocaine considerably diminished postoperative pain, but when
administered only on postoperative period it did not have analgesic
effective results. The mechanisms of analgesia of this
local anesthetic on surgical trauma include neuronal transmission
blockage at the place of injury, reducing neurogenic response
and systemic anti-inflammatory intrinsic activity. Lidocaine’s
analgesic property can persist even after the decreasing of its
plasmatic levels, which corroborates the nervous conduction
blockage theory [6,8-13].
Intraoperatively, aside from analgesia, lidocaine also
promotes reduction of inhaled anesthetics and opioid
consumption, earlier return of bowel function, diminished
production of interleukines and reduction airway reactivity
[9]. This local anesthetic has important anti-inflammatory
properties: reduces cytokine release in vitro and in vivo by
inhibiting neutrophile activation [10,11].
There are few studies with systemic lidocaine use during
the perioperative period and intravenous injection of local
anesthetic is still surprising for many medical professionals,
what aroused interest in the subject chosen for this review.
Methodology
We performed a critical review of literature from March
2011 to March 2014. Articles found on the data base MD Consult
e Clinical Key and published from 1998-2014 were considered.
We used free text and MeSH terms - local anesthetics, pain,
perioperative, intravenou, and lidocaine - for articles in
Portuguese and English language. We recruited additional studies
from bibliographies of retrieved trials and previous reviews. We
excluded data from abstracts, case reports and letters. Of the 463
articles screened, 431 were excluded. A total of 32 articles were
selected, and these were review of the pharmacological aspects
of lidocaine and clinical trials using this local anesthetic for
intravenous continuous infusion perioperatively.
Results and Discussion
Pharmacological Properties
Lidocaine has been used for several indications such as
regional anesthesia, antiarrythmic, on peripheral and central
pain treatment, and as adjuvant on postoperative acute
pain treatment including opioid refractory cases [8]. Recent
researches have shown its mechanism in a more detailed way,
emphasizing its multimodal action.
Lidocaine or 2-(Diethylamino)-N-(2,6-dimethylphenyl)-
acetamide is a weak base, with a pKa of 7,9. In general, local
anesthetics with a pKa that approximates physiologic pH have
a higher concentration of non-ionized base resulting in a faster
onset. Lidocaine itself has a great amount of lipid soluble and
non-ionized local anesthetic on plasma, therefore it has the
property of easily penetrating the neural sheath and axonal
membrane [8,13].
Lidocaine and its metabolites monoethylglycinexylidide
(MEGX), glycinexylidide, and N-ethylglycine, interacts with
peripheral and central voltage-gated sodium channel on intracellular face of membrane blocking the start and conduction
of neural impulses potencial [8].
When intravenously administered, this local anesthetic is
first distributed to highly perfused organs such as brain, heart,
lung, liver and kidney, followed by less perfused tissues like
skin, skeletal muscles, fat and peripheral organs. Its volume of
distribution is great, as 60% of its molecules are bind to plasma
protein [12].
Close to 40% of systemic lidocaine is extracted at the first
stage of the process at the lungs, highly reducing the intoxication
probability after accidental intravascular injection [12]. Its
elimination half-life is of 1,5 to 2h and about 90% of the drug
is metabolized by the liver, at the microsomal enzyme system
(cytochrome P450). Its degradation pathway is mainly the
conversion to monoethylglycinexylidide (MEGX) by oxidative
N-de-ethylation followed by hydrolysis to 2,6-xylidine. Those
metabolites have active properties and have been related to
toxicity cases of systemic local anesthetic after repeated bolus
and continuous infusion. The lidocaine excretion occurs in the
kidneys through an early renal elimination, from 8 to 17 minutes,
and a late phase elimination of 87 to 108 minutes [12].
Mechanism of Action
Lidocaine’s intravenous administration has peripheral and
central action, and involves several mechanisms: sodium channel
and NMDA (N-methyl-D-aspartate) receptors block, glycinergic
action and substance P decrease. In low concentration, it inhibits
primary afferent fibers abnormal activity, mainly at C fibers;
causes sympathetic block, vasodilation and breaks the sequence
of action that perpetuates the painful stimulus. In therapeutic
plasma concentrations (1,5 a 5 μg.mL-1), it diminishes
the hiperexcitability without affecting nerve conduction;
promotes reduction of medular sensitivity and post-synaptic
despolarization NMDA and neurocinine mediated; also reduces
medullary neuron activity [13,14].
Systemic lidocaine has antinociceptive effects in which
glycinergic mechanisms might be involved. Synaptic levels of
glycine, an important inhibitory neurotransmitter, is regulated by
glycine transporters (GlyT1 and GlyT2). In a study that analysed
GlyT1’s function in rats astrocytes and frogs oocytes, the local
anesthetic lidocaine itself, reduced glycine uptake only at toxic
concentrations. However, the metabolites MEGX, glycinexylidide,
and N-ethylglycine significantly reduced glycine uptake at a
clinically relevant concentration increasing extracellular glycine
levels. This increasing of the extracellular level of glycine at the
synaptic cleft via blockade of GlyT1, inhibits the pathologically
increased conduction of excitatory signs in glutamate and
NMDA receptors responsible for the painful stimulus, assuring
antinociceptive effect [14].
Besides of acting at voltage-gated sodium channels, studies
showed that lidocaine yet has effects over G protein-gated, NMDA
and calcium-activated potassium channels receptors, through what it alters the excitatory impulse conduction over A-delta
and C fibers, modifying also visceral pain sensitivity, central
sensitization and immunological response resulting from pain
stimuli [2,15,16].
In other hand, this local anesthetic seems to indirectly block
NMDA receptors through proteinkinase Cinhibition, with impact
over postoperative hiperalgesia and opioids tolerance [17].
When lidocaine is used systemicaly, there is an increasing of
acetylcholine levels at the liquor, exacerbating pain sensitivity
inhibition via descending inhibitory pain pathways, with
consequent analgesia. Related to that, is likely that lidocaine’s
connection with M3 muscarinic, glycine receptors inhibition and
endogenous opioid releasing corroborates to its analgesic final
effect.
Reduction of inflammatory response to ischemia and
diminution of endothelial cytokine-induced tissue damage
through adenosine triphosphate release and potassium channel
is something that also happens. It is wondered that systemic
lidocaine may reduce myocardial ischemia, vasoconstriction
and trombose mediator thromboxane A2 production by directly
interacting with the endothelial membrane [8,13].
Lidocaine interferes in a few inflammatory processes
like oxygen free radicals production, lisosomic neutrofile
sensitization and degranulation, and cytokine releasing at
macrophages and glia cells. It also reduces cytokine induced
cellular damage through mitochondrial potassium channels
adenosine triphosphate sensitive [18,19].
In summary, the mechanism of action of this local anesthetic
is capable of promoting clinically relevant relief of spontaneous
pain, dysesthesia, hiperalgesia and mechanicallodynia through
various pathways [13,20].
Toxicity
As systemic lidocaine’s circulation level increases, the
signs and symptoms of its effects over central nervous and
cardiovascular systems are manifested.
Lidocaine’s plasma concentrations below 5 μg.mL-1 causes
analgesia and inhibition of cortical motoneurons, justifying its
anticonvulsive action [20]. In higher seric levels, from 5 to 10
μg.mL-1, there is perioral paresthesia, metallic taste, dizziness,
diplopia, tinnitus, drowsiness, confusion, agitation, muscle
twitching and seizure. The last one happens with doses between
10 and 15 μg.mL-1 [13].
Many times, seizure is the first sign of severe local anesthetic
toxicity. It occurs because of inhibition of the inhibitory
neurons through GABA (gamma-aminobutyric acid) receptors
stimulation at central amygdala. The seizure usually happens
when lidocaine’s plasmatic concentration is over 8 μg.mL-1,
although it can arise in lower concentrations in hipercarbia
situations [15]. Yet, cardiovascular toxicity goes with depression
of myocardial automatism in lidocaine doses higher than 25 μg.mL-1. It manifests as bradycardia, prolonged PR interval and
wide QRS complex, conduction block, progressive hypotension
and ventricular arrhythmias. Severe cardiac toxicity demands
almost three times the seric concentration that causes seizures.
The treatment of the toxicity must include clinical support
with oxygenation, hydrating and use of vasopressors, inotropic,
antiarrhythmic and anticonvulsivants according to clinical
needs [21]. Implementing lipid therapy is indicated to prevent
cardiovascular collapse based on clinical severity and rate of
progression of symptoms, since only a fraction of patients will
progress to severe toxicity to local anesthetics [22].
Clinical Studies
Systemic lidocaine used in continuous infusion on
perioperative period has analgesic, antihiperalgesic and
antiinflammatory properties, which makes it capable of reducing
intra and postoperative drugs consumption and patients
hospital stay [16,19]. Its effects are mostly pronounced with
intraoperative infusion followed by postoperative infusion of
intravenous lidocaine for days and even weeks, that is long time
infusion and over the drug’s plasmatic half-life applicability. This
indicates that lidocaine’s action is not limited to voltage-gated
sodium channels but it is extended to other goals, and suggests
prevention of hypersensitivity at the central and peripheral
nervous system regularly started and kept by painful stimuli
[6,18].
Lidocaine’s intravenous most appropriate dose for treating
post operative pain in a more efficient way is not yet defined.
Some authors have shown that low doses like in between 1,5 e
3 mg.kg-1.h-1 (plasmatic levels lower than 5 μg.mL-1) reduce pain
after surgical procedures with lower incidence of side effects
and without influence at nerve conduction [15,16,18,23].
Grigoras and colleagues, made a prospective, double blinded,
controlled clinical trial in 36 patients Asa I e II, submitted to
total mastectomy with or without complete axillary dissection.
Of those, 17 received intravenous infusion of lidocaine 1,5
mg.kg-1 in 10 min immediately after orotracheal intubation,
followed by 1,5 mg.kg-1.h-1 stopped 60 min after skin closure.
The 19 other patients received saline solution under the
same scheme. All patients were evaluated for acute pain and
postoperative pain persisting after three months, besides the
extension of secondary hiperalgesia area. As a result, there was
that morfine consumption was alike on both groups during
the first 4h postoperative; plasmatic lidocaine levels were in
the adequate average considering the drug’s toxicity; lower
incidence of postoperative persistent pain and smaller extension
of hiperalgesia area at the surgical incision at the systemic
lidocaine continuous infusion group [24]. In other words, this
study brought to evidence the analgesic and antihiperalgesic
properties of the systemic use of lidocaine via venous infusion
perioperatively, offering better postoperative pain control, what
may also be a way of preventing pain cronification [2].
Koppert [25] and collaborators demonstrated that patients
whom received lidocaine via venous infusion in low doses intra
and postoperatively (bolus of 1,5 mg.kg-1 for about 30 minutes
before surgical incision, followed by continuous infusion of 1,5
mg.kg-1.h-1 until 60 minutes after end of surgery) felt less pain
at mobilization and needed less amount of morfine at the first
72h after abdominal surgery compared to patients that didn’t
receive lidocaine. As this effect of reducing opioid needs was
more evident at the third day of postoperative period, lidocaine
may have a truly preventive analgesic activity avoiding pain
sensibilization and its consequent central induced hiperalgesia
in a clinically relevant way [17].
In a clinical trial made by Kaba and colleagues, lidocaine
was used in patients undergoing laparoscopic colectomy
administered as bolus of 2 mg.kg-1 pre-incisional and kept as
continuous infusion of 3 mg.kg-1.h-1 till the end of the procedure
promoting significant relief of postoperative pain and fatigue,
faster return of bowel function, lower volatile anesthetic and
opioid consumption, reduction of interleukine production (IL-
1AR, IL-6 e IL-8) and of the hospitalization time [16].
Herroeder and collaborators achieved similar results in
a group of 60 patients submitted to colorectal surgery that
refused or had contraindications to epidural catheter. It was
infused intravenously lidocaine bolus of 1,5 mg.kg-1 before
induction of anesthesia, followed by continuous infusion of
2 mg.min-1 till 4 hours after surgery. Lidocaine significantly
decreased return of bowel function period and reduced time of
hospital stay in one day. Besides of that, it was found important
attenuation of increasing of inflammatory markers suggesting
an anti-inflammatory activity and a potential modulating effect
over inflammatory response to surgical stress. There was no
difference in pain evaluation criteria. Nevertheless, systemic use
of lidocaine may be a very convenient and low cost alternative
to get analgesia and satisfactory anesthetic outcomes in patients
that cannot go through epidural anesthesia[26].
Marret and colleagues performed a metanalysis that selected
8 randomized, double-blinded clinical studies that evaluated
a total of 320 patients undergone exclusively to abdominal
surgeries. Of those patients, 161 received intravenous infusion
of lidocaine and 159 received placebo. In 7 of the studies
lidocaine was administered in bolus of 1,5 a 2 mg.kg-1 initiated
before surgical incision, followed by continuous infusion at the
same dose till the end of surgery or 24h postoperative. In the
8 studies evaluated, the result of systemic lidocaine’s use was
reduction of postoperative paralytic ileus duration, pain, nausea
and vomit and time of hospital stay [27].
Saadawy and collaborators made a double-blinded study in
120 patients submitted to laparoscopic cholecystectomy using
the lidocaine dose of bolus of 2 mg.kg-1 followed by continuous
infusion of 2 mg.kg-1.h-1. There was lower need of morfine use at
the second postoperative hour. The lidocaine group had lower scores of abdominal pain at rest and during coughing episodes,
with 2, 6 e 12h postoperative, and faster recovery of bowel
function. At the end of lidocaine’s infusion it’s plasmatic levels
were of 2,6μg.mL-1 [28].
Yardeni and colleagues examined 65 patients undergoing
hysterectomy under general anesthesia. The group that used
lidocaine had 2 mg.kg-1 bolus at anesthetic induction followed
by 1,5 mg.kg-1.h-1 in continuous infusion till end of surgery.
This group presented lower scores of pain at rest and during
coughing episodes at the first 8h postoperative, and attenuation
of immunologic response due to the lower production of
cytokines pro and anti-inflammatory (IL-6 e IL-1ra respectively).
This indicates that perioperatively use of systemic lidocaine
improves acute pain control in immediate postoperative period
and reduces surgical stress-induced immune response [29].
Wongyingsinn and collaborators evaluated 60 patients
undergoing colorectal laparoscopic surgery in which it was used
systemic lidocaine 1,5 mg.kg-1 infusion (maximum of 100 mg) in
anesthetic induction, maintained as 2 mg.kg-1.h-1 infusion until
the end of the surgical procedure and 1 mg.kg-1.h-1 at the first
48h postoperative. The authors compared epidural thoracic
analgesia with general anesthesia and observed that systemic
lidocaine produced similar benefits to return of bowel function
and analgesia’s global quality on patients submitted to colonic
resection. There was no statistical difference at the time of
hospital stay between the evaluated groups [30].
Swenson and collaborators studied 45 patients undergone
to colon resection open surgery and compared epidural
thoracic analgesia using bupivacaine 0,125% and hidromorfone
6mcg.mL-1 10mL/h for 1h until the end of surgery, and
general anesthesia with lidocaine bolus at induction with
approximately 1,5 mg.kg-1 and maintenance according to the
scheme: 1 mg.min-1 in < 70 kg patients and 2 mg.min-1 in ≥70
kg patients. The authors didn’t notice any difference between
the groups related to return of bowel function, time of hospital
stay and postoperative pain control, suggesting once more that
intravenous infusion of lidocaine may be an effective alternative
to epidural therapy in patients that neuroaxial anesthesia is
refused or contraindicated [31].
Kang and colleagues examined 48 patients submitted
to gastrectomy under general anesthesia with intravenous
lidocaine in bolus dose of 1,5 mg.kg-1 at induction and same dose
incontinuous infusion until the end of surgery. This technique
significatively diminished the opioid postoperative consumption
and time of hospital stay, although this study hasn’t shown any
improvement of pain levels and return of bowel function [32].
Most recently, Kyoung-Tae and collaborators evaluated the
effect of intravenous lidocaine infusion on postoperative pain at
lumbar microdiscectomy at a prospective, randomized, doubleblinded
controlled clinical trial with 51 patients. The control
group received lidocaine infusion pre and intraoperative in 1.5 mg.kg-1 bolus followed by 2 mg.kg-1.h-1 infusion until the end
of surgical procedure, and placebo infusion of saline solution.
The lidocaine group had statistically relevant results with low
pain scale scores and lower opioid consumption at first 48h
postoperatively and in the total amount, smaller frequency of
patient controlled analgesia button push, shorter length of time of hospital stay and higher patient’s satisfaction scores. That
is, systemic lidocaine reduced the painful perception during
microdiscectomy, consequently diminishing opioid consumption
and postoperative pain intensity, which contributed to a shorter
hospital stay [33] (Table 1).
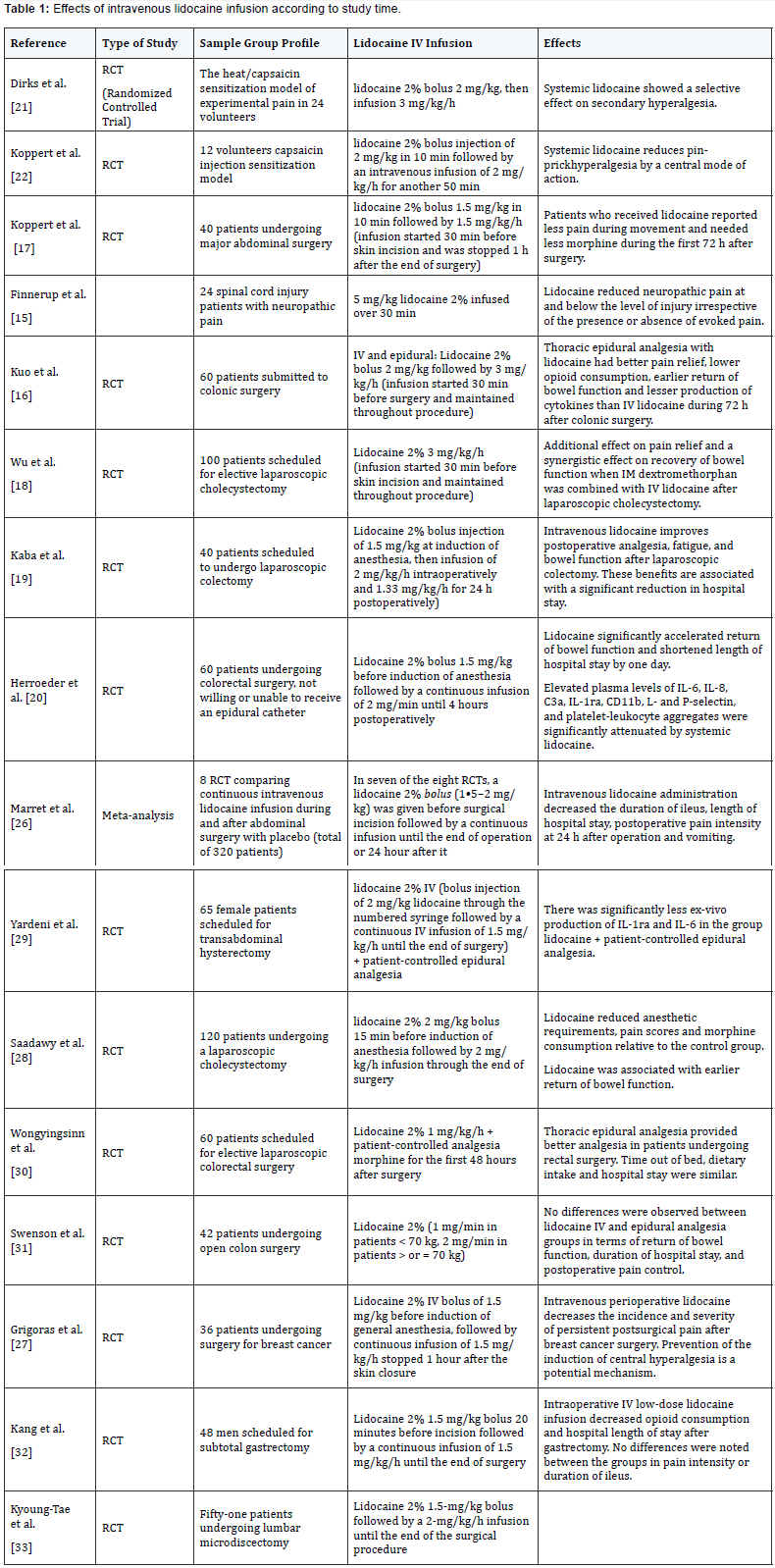
In general, this literature review analysis showed that in
most of the clinical studies selected systemic lidocaine for
perioperative analgesia was used in the dose of 1,5 a 2mg.kg-1
in bolus at anesthetic induction followed by continuous infusion
of 1,5 a 3 mg.kg-1.h-1 intraoperative until the end of the surgical
procedure. It was seen that lidocaine, in this therapeutic form,
produces clinically relevant analgesia intra and postoperatively,
prevents chronic pain, reduces consumption of volatile
anesthetics and opioid, significantly accelerates return of bowel
function and, this way, reduces time of hospital stay. Yet, it came
to evidence that lidocaine causes important attenuation of
production of various inflammatory markers suggesting an antiinflammatory
activity and potencial mechanism of modulation
of surgical stress-induced inflammatory response. All these
findings show that intravenous continuous infusion of lidocaine in the perioperative period may be a convenient and low cost
alternative to achieve analgesia and satisfactory anesthetic
outcomes in patients that cannot undergo epidural anesthesia.
Conclusion
In the past years, the use of systemic lidocaine as analgesic
perioperative technique gained more visibility. This literature
review verified that the dose of intravenous lidocaine with good
clinical outcomes was bolus of 1,5 a 2mg.kg-1 in the anesthetic
induction followed by continuous infusion of 1,5 a 3 mg.kg-1.h-1
intraoperative until the end of the surgical procedure.
It was concluded that the recent studies prove the efficiency
of the use of this local anesthetic on the perioperative period
because of its properties of acute pain relief and chronic pain prevention, besides of reducing the consumption of anesthetics
and promoting early return of bowel function, accelerating
hospital discharge.
This way, systemic lidocaine should be seen as one more
option of analgesia on anesthesiologists antalgic therapy wide
range of medication possibilities. Its administration is low
cost compared to other medications, also more achievable and
clinically safe in posologic well established limits, with specific
indication and good alternative to promote efficient analgesia in
patients that have any contraindication to neuroaxial anesthesia.
The effort of elaborating more controlled clinical studies with the
use of systemic lidocaine in different surgical intervention may
bring more relevant information about this analgesic approach.
For more
details Journal of Anesthesia & Intensive Care Medicine
please
click on: https://juniperpublishers.com/jaicm/classification.php
To read more…Full Text in Juniper Publishers click on https://juniperpublishers.com/jaicm/JAICM.MS.ID.555551.php
Comments
Post a Comment