The Conversion from Continuous Sufentanil Infusion to Oral Retarded Opioid Medication: Beware of the Equi-Analgesic Opioid Ratios - A Case Series-Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Background: Sufentanil has an outstanding
place in clinical practice and one cannot think of surgery or intensive
care therapy without it. However, the routine use of continuous
sufentanil infusion may cause severe problems if stabilized patients are
discharged from the ICU after surgical treatment and need to be
converted to oral opioids.
Aim & method: Here we report our
experiences with a series of six patients that we have converted from
intravenous sufentanil to oral morphine.
Cases: In 6 cases, we report intensive care
(ICU) patients after surgical or medical therapy, who received
sufentanil infusion for analgosedation. The patients were between 45 and
68 years old. It can be demonstrated that the optimal dose of
sufentanil can be converted to minor doses of oral medication than
expected from the calculated equi-analgesic ratios. Despite of lower
oral opioid medication pain levels did not increase after conversion.
Conclusion: We recommend to begin opioid
conversion with 10% of the calculated equivalent dose of intravenous
sufentanil when converting to oral long-acting morphine and afterwards
to further adapt the dosage.
Introduction
Since its development in the late 70s, sufentanil has
an outstanding importance in clinical practice and one cannot think of
surgery or intensive care routines without this treatment. The substance
delivers a much higher potency than its parent drug fentanyl with an
expanded therapeutic range [1,2].
From the beginning of its clinical use, sufentanil was the intravenous
opioid of choice for hemodynamically instable patients [3].
Due to its outstanding hemodynamic stability resulting from a minor
impact on cardiac index, left ventricular ejection fraction and heart
rate [4],
sufentanil is broadly used for critically ill patients in cardiac and
non-cardiac surgery. In comparison with fentanyl, it has a shorter
context-sensitive half time that results in better controllability [5] and predisposes the use of sufentanil in extended cases and for continuous infusion in intensive care.
The decoupling of analgesia and respiratory depression [6]
is another reason for preferring sufentanil during weaning of
mechanically ventilated patients or in those with spontaneous breathing.
However, the routine use of continuous sufentanil analgosedation in the
ICU may result in the problem that stabilized patients are still not
free of pain or suffer from chronic pain and thus need to be converted
to oral opioid medication, if discharged from the ICU after surgical or
medical therapy. For example, common dosage of 20μg of sufentanil per
hour has to be substituted by oral opioids as the patient should be
transferred to the floor. The calculated equivalent dose for oral
substitution would be 1440mg morphine per day, which is, of course, not
practicable.
The following cases should demonstrate that
sufficient pain therapy can be achieved also with significantly lower
morphine doses. We report here six cases in which the hospital pain
service was consulted to assist non-anesthetic intensive care units in
the conversion from intravenous sufentanil to oral medication.
Case Presentation
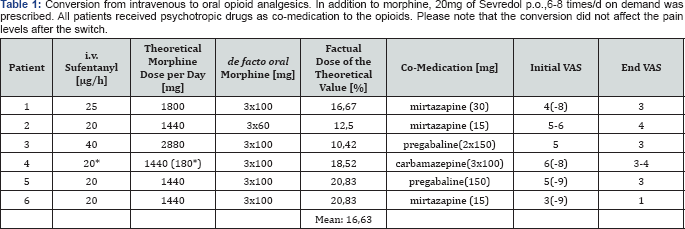
Case 1: Patient J.S., male, 44 years old, weight 170kg, height 175cm; septic shock with multi-organ failure
The patient who suffered from arterial hypertension,
atrial fibrillation, type-II-diabetes mellitus and morbid adipositas was
admitted due to severe and rapid deterioration of his general
condition. He developed a septic shock with subsequent multiorgan
failure including renal insufficiency requiring dialysis, and liver
failure. Furthermore, he developed a cardiogenic shock with a left
ventricular ejection fraction of about 10%, and required
cardio-pulmonary resuscitation (CPR) as ventricular fibrillation
occurred.
After improvement and when the patient was able to be
transferred to the floor, he received sufentanil infusion with 25μg per
hour. The patient reported pain scores between NAS four and eight with
burning quality. Pain therapy was converted orally to long-acting
morphine (MST®, Mundipharma Ltd., Limburg an der Lahn, Germany) 3x100mg
and 30mg mirtazapine (REMERGILSolTab®, MSD Sharp & Dohme GmbH, Haar,
Germany) in the evening and short-acting morphine(Sevredol®,
Mundipharma Ltd., Limburg an der Lahn, Germany), 20mg up to six times
daily on demand. After a stepwise reduction of the morphine dose down to
3x30mg long-acting morphine per day and 30mg of mirtazapine, the pain
service could sign off after seven days.
Case 2: Patient P.M., male, 63 years old, weight 97kg, height 180cm; serial rib fractures with pleural empyema
*This patient received additionally transdermal fentanyl (Durogesic SMAT 75pg/h)
The patient suffered from a traumatic left-sided rib
series fracture and developed pneumonia and a pleural empyema while
under conservative therapy. Secondary diagnoses comprised arterial
hypertension, COPD, type-II-diabetes mellitus and chronic renal
insufficiency. After surgical intervention and intensive care therapy
with prolonged weaning, the patient was presented to the pain service
for conversion to oral opioids. The current pain therapy was 20μg/h of
i.v. sufentanil (Table 1).
The patient was switched to 3x60mg long-acting morphine sulphate (MST®,
Mundipharma Ltd., Limburg an der Lahn, Germany) and 15mg mirtazapine
(REMERGIL SolTab®, MSD Sharp & Dohme, Haar, Germany) in the
evenings; additionally Sevredol® 20mg up to eight times daily was
prescribed, if VAS exceeded 5. After a stepwise reduction of the
morphine dose down to 3x30mg with an evening dose of 15mg mirtazapine,
pain service consultation ended after four days, the patient being
satisfied at VAS <4.
Case 3: Patient S.L., female, 53 years old, weight 146kg, height 170cm; sepsis with multiple arterial emboli
The patient was primarily treated for a sepsis with
unknown focus and suffered from morbid adipositas, a history of
hypertension and type-II-diabetes mellitus in the intensive care unit.
During the clinical course, both legs had to be partially amputated due
to multiple arterial emboli; the right leg below the knee, the left leg
above.
Under sufentanil infusion of 40μg/h, the patient was
presented for conversion to oral therapy. The initial regime comprised
3x100mg of long-acting morphine with pregabaline (Lyrica®, Pfizer®,
Berlin, Germany), 2x150mg, and Sevredol®, 20mg up to 6 times daily, if
VAS exceeded 5. The consultation ended after five days, with morphine
dosage reduced to 3x30mg of long-acting morphine and pregabaline
2x150mg. The patient was satisfied at VAS <3.
Case 4: Patient K.K., male, 58 years old,
weight 104kg, height 180cm; osteomyelitis and acute renal failure after
coronary arterial bypass grafting (CABG) surgery
The patient was treated for sternal osteomyelitis and
acute renal failure after coronary arterial bypass grafting. In
addition, the patient suffered from arterial hypertension, peripheral
arterial vascular disease, hyperlipoproteinemia, COPD (GOLD III) and had
been treated previously for laryngeal cancer with laryngectomy and
bilateral neck dissection. At presentation to the pain service for
conversion to oral medication, the patient received 20μg/h sufentanil
with additional transdermal fentanyl (Durogesic SMAT 75μg/h,
JANSSEN-CILAG, Neuss, Germany), which the patient had already before
surgery. Pain scores of VAS=6 with peaks at VAS=8 were reported. The
patient was converted to long-acting morphine 3x100mg/day and
additionally with 3x100mg carbamazepine (Carbamazepin HEXAL®, Salutas
Pharma, Barleben, Germany) with opportunity of receiving supplementary
20mg Sevredol®, up to 8* per day. After reducing long-acting morphine to
2*50mg with carbamazepine 3*300mg, pain service consultation ended
after six days, the patient being satisfied at VAS=3-4.
Case 5: Patient R.S., male, 66 years old, weight 80kg, height 178cm; Multiple Myeloma and ARDS
The patient needed mechanical ventilation support for
acute respiratory insufficiency under pre-existing multiple myeloma.
During the clinical course, the patient developed acute renal failure
requiring dialysis, aspiration pneumonia and critical illness
polyneuropathy. After prolonged weaning, an apparently pain stricken
patient was presented to the pain service receiving 20μg/h sufentanil,
for conversion to oral analgesics.
At pain levels of VAS=5 and peaks of VAS=9, initially
long- acting morphine 3*100mg/day with 150mg pregabaline (Lyrica®,
Pfizer, Berlin, Germany) in the evenings was prescribed, with the
possibility of additionally receiving 8*20mg Sevredol® per day. After
stepwise reduction of morphine dose to 2*20mg/d of long-acting morphine
and 150mg pregabaline in the evenings, the patient was discharged from
the ICU with VAS=3 and the patient was discharged with 2*10mg/d long-
acting morphine and with 150mg pregabaline.
Case 6: Patient K.B., male, 62 years old, weight 60kg, height 160cm; hemorrhagic shock after bypass surgery of the femoral artery
Following bypass surgery of the femoral artery with
secondary hemorrhage and hype volemic shock, the patient developed an
urosepsis. Preexisting diagnoses were peripheral vascular disease,
arterial hypertension, type-2-diabetes mellitus and stage-III-renal
insufficiency. After stabilizing the patient and planning for discharge
to the ward, pain service was consulted for conversion of i.v.
Sufentanil, 20μg/h, to oral medication.
The patient described pain as having
piercing/stabbing qualities at VAS=3, peaking at VAS=9. After a stepwise
reduction of initially 3*100mg/day long-acting morphine with
mirtazapine 15mg for the night, the patient was discharged from the ICU
with 3*60 mg/d long-acting morphine with afore mentioned mirtazapine at
VAS=1.
Discussion
In clinical practice, sufentanil is indispensable for
anesthesia and intensive care therapy. However, a conversion from
continuous sufentanil infusion to oral opioid medication is essential
for discharge from the ICU; however, current literature offers no usable
conversion algorithms.
The pain levels of a series of six patients presented
here indicate that opioid conversion to lower oral doses does not
result in an increase of pain scores. Additionally administered
psychotropic drugs may also have an effect on alleviating pain, yet two
aspects have to be taken into account: (1) pain aggravation by under-dosing of opioids cannot be compensated by psychotropic medication, and (2)
if the opioid dose is titrated to an optimum, psychotropic drugs cannot
further reduce this dose. They can only be used to avoid severe side
effects of opioid therapy [7].
In the present cases, psychotropic medication was used to treat effects
of opioid over-dosing after conventional conversion, and was needed to
treat the neuropathic aspects of the respective pain qualities [8].
It is important to note that the conversion to oral
opioids is not an "opioid rotation", although one has to calculate an
equi- analgetic dose. The concept of opioid rotation addresses the
problem of excessive side effects [9] of a single opioid or the insufficient effect on pain [9,10].
This was not the case in the presented patients. In those, we intended
to switch an i.v. opioid to an orally applied one, much in the way a
morphine drip is switched to oral retarded morphine.
Sufentanil is available as a non-i.v. preparation for
sublingual, buccal and nasal administration but not in a long- acting
formulation. As the application route switch is usually for a single
compound and the long-acting formulation is commercially unavailable,
change to long-acting morphine was necessary, but not in the sense of an
opioid rotation.
In current references, only the general
recommendation to begin oral substitution with approximately 50% of the
equivalent dose can be found [10,11].
These recommendations are based on the thought that on one hand the
patients have not benefitted from the current opioid and on the other
they offer concomitant clinical limitations (i.e. advanced age, renal
damage, cardiopulmonary insufficiency, etc.) that makes a 1:1 switch to a
new opioid inappropriate.
The patients in the presented cases had an i.v.
sufentanil medication near the optimum dose. The available conversion
tables and factors suggested a 900% higher dosing than that we
eventually applied. Even with a reduction of 50% from the given i.v.
dose, the orally administered amount would still have been in excess of
350% of the dose that is finally necessary. This is striking, as
inadequately high doses of opioids can lead to severe side effects such
as attention deficits, optical hallucinations and ultimately respiratory
depression [12,13].
From the present data, we provide evidence that, when
converting i.v. sufentanil to oral morphine, a much steeper reduction
of the equivalent dose is urgently warranted.
We would like to recommend starting with 10-20% of
the calculated equivalent dose of sufentanil infusion when converting to
oral long-acting morphine and afterwards adapting the morphine dosage
further. Possible co-medication with neuroleptics and benzodiazepines
should not be ignored in order to further minimize opioid doses and to
decrease severe side effects.
In the possible case that the conversion to a
long-acting opioid proves insufficient, a similar approach as usually
followed in opioid conversion should be used: In addition to the
estimated dose, rescue medication needs to be provided. This can be
claimed every hour by the patient and, in the case of using morphine
sulfate, doses of 10mg and 20mg with an onset of 15 to 20 minutes should
be available. It seems important that none of our patients claimed
rescue medication.
Conclusion
Owing to safety considerations, we propose to
approach the final opioid dose from a lower dose. By doing this, severe
side effects and a possible readmission to the intensive care unit can
be avoided. Moreover, since the increased pain perception precedes
withdrawal symptoms, correcting the opioid dose in an hourly interval
would not have led to withdrawal indicators [14-18].
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php
Comments
Post a Comment