Narrowing the Gap between the Anion Gap and the Strong Ion Gap-Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Background: Despite its importance in
understanding acid-base pathophysiology, many physicians do not
comprehend the concept of the strong anion gap (SIG), the core of the
Stewart acid-base approach. The quantitative difference between the
anion gap and the strong ion gap is not established. This paper will
give insight into this difference over a variety of conditions Methods.
The empiric difference between the SIG and the albumin-corrected anion
gap (AGc) was calculated at a wide range of albumin, phosphorus and pH
levels.
Results: At an albumin level of 1-3 g/dl and pH from 6.9-7.3, the contribution difference of albumin between the AGc and the SIG will be maximally -0.97 to 0.51mEq/L. In metabolic alkalosis and hypoalbuminaemia, the AGc
differs less than 2mEq/L from the SIG. The calculated contribution of
phosphorus is higher in the SIG with phosphorus levels >2mmol/L and
can be accounted for in the anion gap with the conversion factor 1.76*
[phosphorus, in mmol/L].
Conclusion: The SIG and the AGc are
nearly identical across a wide range of values, particularly when
albumin and phosphorus levels are low. The anion gap will be more
precise and incorporate the major components of the SIG using the
equation: [Na+]-[Cl-]-[HCO3-]-2.5* [albumin, in g/dL] - 1.76* [phosphorus, in mmol/L], with an arbitrarily set reference range of 1±5mEq/L.
Keywords: Acid-base; Anion gap; Strong anion gap; StewartAbbreviations: AGc: Corrected Anion Gap; AlbSt: Albumin Measured By The Stewart Formula; Pst : Phosphate Measured with the Stewart Formula;AGa+p : AG Corrected for Albumin and Phosphorus
Introduction
For more than 40 years clinicians have used the anion
gap (AG) as a major tool to evaluate acid-base disorders. A positive
value of the AG suggests a possible organic acidosis due to endogenous
acids or the intake of exogenous acids [1]. Although the concept of the AG was described in 1936 by James Gamble [2].
it did not gain widespread recognition by physicians until the 1970s
after the introduction of auto analyzers and the rapid availability of
multiple analytes. According to Gamble, electrical neutrality in
solution demands that the sum of the cations is equal to the sum of the
anions, also represented in a Gamblegram (Figure 1).
Sodium, chloride, bicarbonate and albumin are quantitatively the major
ions in the extracellular fluid compartment and are therefore used to
calculate the anion gap. A true "ion gap" does not exist in vivo which
makes the anion gap a fundamental tool to evaluate acid-base disorders [1].
In 1978 Peter Stewart challenged the traditional bicarbonate- based
approach, by reasoning that his approach offered both a mechanistic
explanation and provided the tool to make a more accurate diagnosis [3]. The core of the Stewart approach is the strong anion gap (SIG): Equation 1.

[Na+] + [K+] + [Ca2+] + [Mg2+]-[Cl-]-[lactate-]-12.2xpC02/ (10'pH)-10x[albumin]x0.123xpH-0.631)-[PO4- in mmol/ L]x(0.309xpH-0.469)= +0mEq/L
This complex formula quantitatively accounts for the contribution of weak acids to the electrical charge equilibrium in plasma [3]. On first glance there is a substantial difference from the AG equation: Equation 2.
[Na+]-[Cl-]-[HC03-]=+10mEq/L
Though the SIG gives a wider-ranging picture of the
ion balance than the AG, controversial opinions about its real value
remain and several differences are observed between the SIG and the AG:
- The SIG is popular amongst intensivists, though additional parameters can increase variability and error [4,5]. Additionally, small changes in K+, Ca2+ and Mg2+ are rarely of consequence due to narrow variations in health and disease. Nevertheless, some clinicians include K in the AG. Likewise, one can include Ca and Mg in the AG. The AG may therefore be written like [Na+]+[K+]+[Ca2+]+[Mg2+]-[Cl‘ ]-[HC03-]; the reference value should increase accordingly. These kations are included in SIG (equation 1). When concentrations of these cations are changed substantially one may include them in the AG as well. Therefore, these cations are not relevant for the discussion.
- Lactate may be included in the SIG, but clinicians will typically know that a high AG or SIG is caused by lactate.
- The formula 12.2xpCO2/(10-pH) is used by blood gas devices to calculate the bicarbonate concentration and may be therefore replaced by it. This leaves us with the following equation which starts to approximate the more familiar anion gap: Equation 3.
- Thus, the "only" real difference between the SIG and the AG is that albumin and phosphorus are adjusted for changes in pH in the SIG. The purpose of this study is to determine the significance of this difference across a wide range of conditions.
[Na+]-[Cl-]-[HC03-]-10x[albumin]x(0.12 3xpH-0.631)- [P04]x(0.309 pH-0.469)=+0mEq/L.
Methods
The quantitative difference between the SIG and the AGc
was calculated at a wide range of albumin (from 1 to 5 g/L), phosphorus
(from 0.5to4mmol/L) and pH levels (from 6.9to7.6) with otherwise
standard parameters. For example, with an albumin of 4 g/dl and a pH of
6.9, the albumin effect on the anion gap will be 10mmol/L regardless of
the pH. The SIG will change using the formula
10x[albumin]x(0.123xpH-0.631), by 8.71mmol/L. Thus, the Δ
AlbSt-10=8.71-10=-1.29mmol/L. Similarly, with a phosphorus of 1mmol/L
and a pH of 6.9, the PSt=[P04-]x(0.309xpH-0.469)=1.66mEq/l difference
with the AG.
Results
At an albumin level of 1-3 g/dl and a pH from
6.9-7.3, the contribution difference of albumin in the AGc and the SIG
will be maximally -0.97 to 0.51mEq/L, suggesting little difference
between the two methods (Table 1).
In metabolic alkalosis and hypoalbuminaemia, once again the AGc differs
less than 2mEq/L from the SIG. Incorporation of phosphorus in the SIG
is significant and there is a linear relationship of the serum
phosphorus and the ionic contribution in the SIG (supplemental data).
This effect will therefore be more notable at higher phosphorus levels (Table 1).

AlbSt = albumin concentration calculated by the Stewart method: 10 * [albumin] * (0.123 * pH - 0.631).
PSt = phosphorus concentration calculated by the Stewart method: [PO4- in mmol/L] * (0.309 * pH - 0.469).
Δ Alba - albumin at a certain level
calculated by the Stewart method minus the albumin concentration
adjusted for the corrected anion gap method (2.5 g/dL for each g/dL
change). Example: with albumin = 4 g/dl at a pH = 6.9 the albumin effect
on the anion gap will be 10 mmol/L (regardless of the pH in the anion
gap concept). The SIG will change using the formula 10 * [albumin] *
(0.123 * pH - 0.631), by 8.71 mmol/L.
Δ AlbSt - 10 = 8.71 - 10 = -1.29 mmol/L.
Δ PSt phosphorus concentration at a
certain level calculated by the Stewart method minus the phosphorus.
Example: If the phosphorus is 1 mmol/L and the pH is 6.9, the change in
SIG will be 1.66 mmol/L using the formula: {[PO4-] * (0.309 * pH - 0.469)}. The pH adjusted level A
PSt - 1 = 1.66 - 1 = 0.66 mmol/L.
⊗PSt-1 = 1.66 - 1 = 0.66 mmol/L.
aReference range albumin (3.5-5.5 g/dL)
bReference value phosphorus: 0.97-1.45 mmol/L
Discussion
Body fluid compartments have varying concentrations
of non-volatile weak acids, with albumin and inorganic phosphorus the
primary components in plasma [3]. The anion gap is used to evaluate metabolic acidosis and is calculated as follows:
[Na+]-[Cl-]-[HC03-]
Because of the narrow extracellular concentration, K+
is often omitted from the calculation. To be more precise, one should
correct the anion gap for hypoalbuminemia. To appreciate the relevance
of this correction one can consider a healthy individual with the
following serum values:
[Na+]=140mEq/L, [Cl-] = 106mEq/L, [HC032-]=24mEq/L.
The AG=10mEq/L, representing primarily albumin. The correction factor for albumin is 2.3-2.5 * [albumin], in g/dL [1]. The albumin corrected AG (AGa) equation can therefore be written as: Equation 4.
[Na+]-[Cl-]-[HC032-]-2.5 [albumin, in g/dL]=0+5mEq/L.
This equation gives a better understanding of the
principle that there is no actual "gap" between the positive and
negative ions and likewise, the "AG" should be zero in-vivo.
However, comparing this formula with the SIG, one will observe that a
major difference is the inclusion of phosphorus in the SIG and that
albumin and phosphorus are both adjusted for changes in pH. The question
is therefore if one should include phosphorus as well in the AG.
Albumin
Hypoalbuminemia is common in patients with acid-base
disorders. With an albumin of 1-3 g/dl, the contribution difference of
albumin between the AG and the SIG will be maximally -0.97 to 1.6mEq/L
regardless of pH (Table 1).Therefore,
the pH correction of albumin used in the SIG formula is irrelevant in
the evaluation of metabolic acidosis, as long as the AG is adjusted for
albumin.
Phosphorus
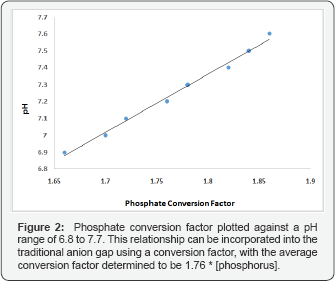
When phosphorus levels are normal or reduced, there is little contribution (<2mmol/L) to the AG (Table 1).
If phosphorus levels are elevated, as in renal failure, the ionic
contribution, however, becomes more significant. Suppose we have a
patient with renal failure and the following plasma electrolytes: [Na+]=140mEq/L, [Cl-]=106mEq/L, [HC032-]=24mEq/L,
albumin 4g/dL, phosphorus of 6mmol/L (reference value 0.97-1.45
mmol/L), and a pH of 7.0. The anion gap corrected for albumin will be
zero. When using equation 3 the SIG will be 6.78mEq/L due to the high
phosphorus level. The linear relationship of serum phosphorus and the
ionic contribution in the SIG can be incorporated in the traditional AG
formula (Figure 2). The SIG can thus be replaced by the following adjustment to the AG formula: Equation 5.
AG corrected for albumin and phosphorus (AGa+p)=[Na+]-[Cl- ]-[HCO3-]-2.5[albumin,
in g/dL]- 1.76 [phosphorus, in mmol/L] and as the median phosphorus
level is about 1.2, the reference value will be about -2+5mEq/L. A
similar equation has been proposed by Kellum et al. [4].
Conclusion
In conclusion, the SIG and AG are almost identical in
a variety of physiological acid-base conditions. When phosphorus levels
are elevated, one can use the less complex AGa+p equation as
an accurate representation of the SIG. To have a better understanding
of the pathophysiology and to be more accurate, the anion gap, or
perhaps a more logical term, "the ion gap" should be written as equation
5 to become almost identical to the SIG.
Acknowledgment
We are indebted to Jan Williem boldingh for malimg Figure 1.
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php
Comments
Post a Comment