Anaesthetic Recommendations for Stiff Person Syndrome-Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Background: Stiff person syndrome (SPS) is a neurological condition with serious implications during anaesthesia if overlooked.
Objective: Our purpose was to highlight the
issues encountered during anaesthesia in patients with SPS and to
evaluate the most appropriate anaesthetic management during surgery.
Methods: A structured search was performed
throughout various databases such as Ovid medline, Pubmed, Embase and
Cochrane Collaboration for articles published from 1950s and after. The
search included only humans and those who underwent procedures with
anaesthesia.
Results: Only 13 of such case studies were
found due to the rarity of the condition. Amongst these 13 patients,
most were in the middle to elderly aged groups and they underwent
different procedures. Their response to different drugs used for
induction, neuromuscular blockade or maintenance of anaesthesia also
varied with some leading to life-threatening complications especially
due to postoperative hypotonia. Route of anaesthesia also had a role in
changing the outcomes.
Conclusion: SPS is challenging to manage and
the use of drugs like midazolam, propofol, rocuronium is recommended to
improve patient outcomes. Volatile agents are safe if their doses are
kept to a minimum with bispectral index monitoring. Alternative routes
of anaesthesia may be better but this is not always feasible.
Keywords: Stiff person syndrome; General anaesthesia; Neuromuscular blockers; Inhalational anaesthetics; Surgery Introduction
Stiff person syndrome (SPS) is a unique neurological disorder first described in 1956 by Moersch & Woltman [1]. It has no known genetic predisposition [2] but an association with certain HLA genes exists [3]. SPS is slowly progressive with insidious onset, which commonly begins in the middle-age [4-6]. It is typified by stiffness and rigidity of the axial and proximal limb musculature, with superimposed painful spasms [2,3,6]. In more severe cases, the muscles of respiration and swallowing are affected and may cause respiratory distress [5,7].
The painful spasms can be triggered by external sensory stimuli, voluntary movement, fear, anxiety [8,9]
and appear to arise from the brain or spinal cord because they stop
during general anaesthesia (GA) or sleep. The aetiology of SPS seems to
be autoimmune as circulating autoantibodies reactive with glutamic acid
decarboxylase (GAD) have been found in SPS patients [10,11].
GAD is an enzyme necessary for the production of gamma-aminobutyric
acid (GABA). GABA has an inhibitory (gabanergic) input to muscles
causing a muscle relaxant effect. Reduction in GABA production upsets
the balance of excitatory inputs in various areas of the brain,
specifically the gamma- motor neuron system, leading to continual motor
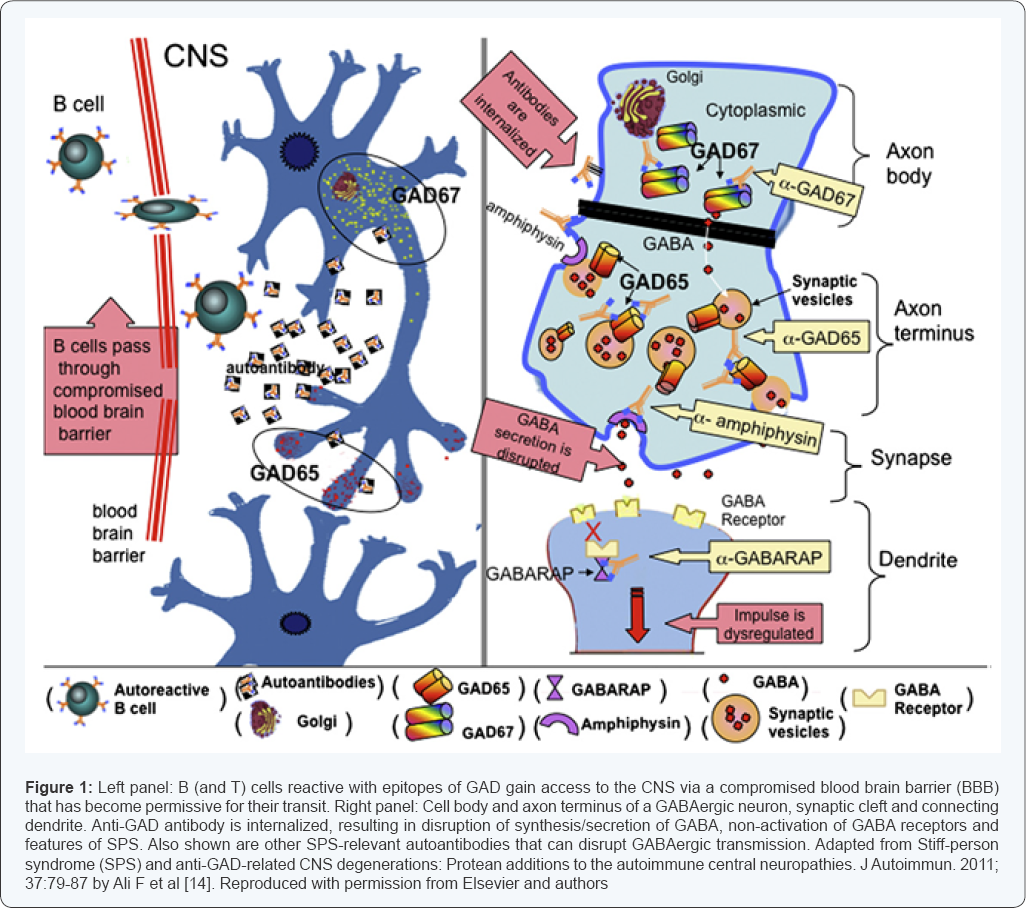
neuron activity and spasms [12,13]. Figure 1 illustrates the action of anti-GAD antibodies [14].
Drugs enhancing central gabanergic neurotransmission
such as diazepam are used in treating SPS because it increases the
frequency that GABA receptors are activated. Baclofen synergises with
diazepam and is prescribed together. Similarly useful drugs include
clonazepam and sodium valproate [5,15]. Other treatments inducing remission involve immunosupression with steroids [5] or rituximab [16].
SPS if overlooked may lead to serious problems with anaesthesia. The mechanism of rigidity involved is different from
malignant hyperthermia which has a rapid onset of symptoms often triggered by various anaesthetic agents [17,18] and SPS should not be a contraindication for surgery. Careful monitoring and choice of anaesthesia is still essential.
The principal aim of this review is to provide
recommendations in the anaesthetic management of a patient with
Stiff-Person Syndrome (SPS) during surgery. Additionally, a literature
review and suggestions on how to choose anaesthetic drugs in order to
avoid postoperative complications will be discussed.
Methods
A structured search was performed through Ovid
medline, Pubmed, Embase and Cochrane Collaboration; this includes
articles in English language published from 1950s until the present. A
Medical Subject Headings (MeSH) search within titles and abstracts with
keywords such as "Stiff man syndrome", "Stiff person syndrome", "SPS",
"SMS", "Anaesthesia", 'Anesthesia", "Anaesthetics" and "Anesthetics" was
done.
The search was limited to include only studies involving humans.
Results
There are no randomised controlled trials or
case-control studies involving the effect of anaesthetic drugs in SPS
patients or recommended anaesthetic drugs of choice. Nevertheless, there
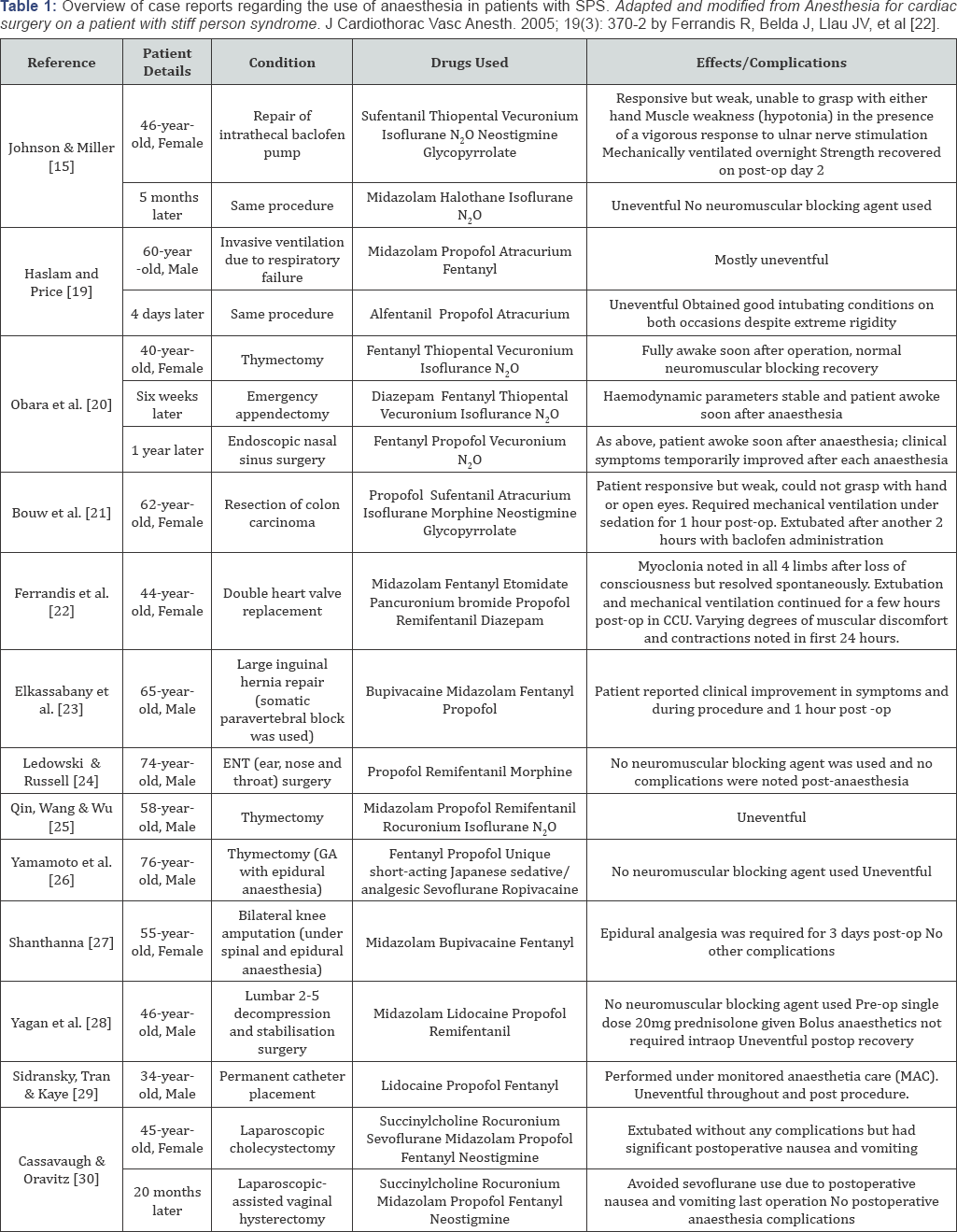
were 13 case reports describing peri- and intraoperative management of
SPS patients; with some studies highlighting the increased risk of
postoperative hypotonia as shown in Table 1 below.
Discussion
Firstly, the use of appropriate temperature
monitoring and regulation during surgery for SPS patients with the aid
of a Bair hugger is recommended. As baclofen is the mainstay therapy for
SPS, such patients would have poor temperature perception. Oliviero et
al. [31]
showed that long-term baclofen therapy would impair temperature
perception as it increases both the threshold for warm and cold stimuli.
This occurs because baclofen activates receptors for GABA-B which
modulates temperature perception.
For induction, propofol is a safe choice and other alternatives include halogenated gases, thiopental, propofol and etomidate [32]
which act via the gabanergic pathway. Such agents should reduce
postoperative muscle spasm or pain in SPS patients, who have decreased
gabanergic input. Etomidate has been reported by Ferrandis et al. [22]
to have a higher risk of intraoperative myoclonus. This reflects
neuromuscular hyperexcitability but it is unclear whether SPS
perpetuated the myoclonus [22], or if etomidate had an idiosyncratic effect. Diazepam, fentanyl and atropine have been prescribed as pre-medication [33] to overcome myoclonus in non-SPS patients but Ferrandis et al. [22]
reported that these were ineffective in their SPS patient. A higher
dose of benzodiazepine could have been useful but there is insufficient
literature to support this [22].
Propofol has also been implicated in case reports to cause intraoperative myoclonus [34-36] but it has a lower risk. A randomised controlled trial by Miner et al. [37]
reported that 18.2 % more (95% CI 10.1% to 26.2%) non-SPS patients who
received etomidate had intraoperative myoclonus compared to those on
propofol. Moreover, propofol improves postoperative muscle rigidity and
spasms as reported by Obara et al. [20]. The published literature suggests that propofol reduces spinal activity [38] by acting primarily on GABA-A receptors [39-41] with some partial effect on central GABA-B receptors [42-44] unlike baclofen, which is a selective GABA-B receptor agonist [45-47].
Propofol would be the drug of choice for anaesthesia
induction in SPS patients with the concomitant use of midazolam, which
also modulates GABA-A receptor activity [43]. Pre-medication is not necessary since these SPS patients would have already been managed with diazepam and baclofen.
Muscle rigidity in SPS patients can make it difficult to position a patient for intubation [19]
during general anaesthesia. Thus there is a role for neuromuscular
blocking drugs. However, Johnson and Miller reported that the use of
vecuronium, (a non-depolarising neuromuscular blocking drug) caused
postoperative hypotonia [15].
This led to their patient requiring mechanical ventilation for 48 hours
despite attempts to reverse the neuromuscular blocking drug. This
complication was not observed by Obara et al. [20], Haslam & Price [19].
Haslam and Price used atracurium but did not encounter similar
complications as described by Johnson and Miller. Johnson and Miller
reported that the mechanism behind their patient's postoperative
hypotonia was unclear but claimed that it did not occur when they
performed the same procedure without neuromuscular blocking drugs. Their
patient also had a past surgical history of prolonged postoperative
weakness when she had her baclofen pump inserted but this was attributed
to baclofen overdose [15].
This particular patient could be idiosyncratic for
having very sensitive GABA-B receptors. Moreover, the dose of thiopental
used in their patient was 5.8mg/kg compared to Obara et al. [20] who only administered 3mg/kg even though they used the same amount of vecuronium (8mg) in their respective patients [15,20].
The additive effect of using a higher dose of anaesthesia at induction
combined with their patient's idiosyncrasy could explain the cause
behind the complications that Johnson and Miller experienced.
Anti-GAD antibodies have no action at the neuromuscular junction, according to Figure 1 [14], hence, neuromuscular blocking drugs would not potentiate their effect. Obara et al. [20]
confirmed that their patient achieved a 25% twitch recovery time (which
was within their patient's normal range) while on vecuronium. Their
patient's train-of-four (TOF) ratio (which indicates depth of
neuromuscular blockade) monitored in the ulnar nerve also recovered to
100% postoperatively. This suggests that the neuromuscular junction was
not affected by SPS [20]. Yamamoto et al. [26]
who did not administer neuromuscular blocking drugs as they were using
epidural anaesthesia in conjunction with GA, also ascertained that the
neuromuscular junction was unlikely to be affected in SPS as their
patient’s TOF ratio remained above 90% throughout the anaesthesia.
Ferrandis et al. [22]
found that the pharmacodynamics of neuromuscular blocking drugs in SPS
was not clearly described in literature. They added that the effect of
neuromuscular blockers used in the cases reported by Johnson and Miller,
Obara et al. [20] was neither greater nor longer lasting than normal. In contrast, Ferrandis et al. [22]
reported longer response and recovery time to the TOF after the
administration of a second dose of 4mg of pancuronium, before ending
extracorporeal circulation for cardiac surgery. They had earlier given
8mg of pancuronium for intubation with normal response and recovery time
to the TOF. Ferrandis et al. [22]
claimed that extracorporeal circulation could possibly have prolonged
the effect of neuromuscular blocking agents. It could also explain why
their patient was mechanically ventilated for a few more hours
postoperatively.
There does not appear to be any anaesthetic
interactions or additional contraindications to use neuromuscular
blocking agents in SPS patients. A recent case reported by Cassavaugh
and Oravitz had successfully managed a patient with both
depolarising and non-depolarising neuromuscular blocking agents on
separate procedures with TOF monitoring [30].
The patient did not experience any postoperative complications. Careful
individual monitoring of neuromuscular response in the form of TOF is
more important and rocuronium/ vecuronium would be the recommended drug
of choice which can be reversed promptly by sugammadex even in
situations of profound neuromuscular blockade [48].
Regarding the hypotonia in Johnson and Miller's patient, Bouw et al. [21] attributed this to baclofen amplifying the gabanergic effects of volatile agents during GA. Bouw et al. [21]
reported that their patient who was usually on baclofen developed
muscle weakness and required mechanical ventilation for 1 hour
postoperatively and suggested the 0.6-1.0% isoflurane as the cause.
Similar postoperative complications were described in a patient who did
not have SPS and received the same drugs [49]. Animal studies have also proved that baclofen potentiates the effects of halogenated agents [50]. Volatile anaesthetics enhance gabanergic input by extending postsynaptic inhibitory currents when GABA is released [51,52]. Since SPS patients are on baclofen for treatment, the doses of such anaesthetic agents should be adjusted. Bouw et al. [21]
also confirmed on pharmacokinetic analyses that neuromuscular blocking
agents and opioids did not play a role in the complications observed.
Not all reported cases had significant adverse
outcomes; Cassavaugh and Oravitz did not encounter any respiratory
problems with sevoflurane use [30].
Qin, Wang and Wu also reported a case of paraneoplastic SPS requiring
thymectomy which did not develop any prolonged postoperative hypotonia
or weakness. They attributed this mainly to the low concentration of
isoflurane used (0.2-0.4%) as they used a target-controlled infusion of
remifentanil and nitrous oxide for maintenance
[25]
. This is a useful method of minimising the concentration of volatile
agents but it is not tailored to individual patients. A better strategy
to overcome this was reflected in the case reported by Yamamoto et al. [26]
who used the bispectral index (BIS) to monitor the minimum
concentration needed. These gases may cause postoperative hypotonia when
used in combination with ongoing baclofen therapy but their
concentration can be kept to a minimum with BIS monitoring. This
approach would allow the continued use of these agents with reduced risk
of respiratory failure.
Different routes of anaesthesia may help as suggested by Yamamoto et al. [26] and Shanthanna [27]
who both used epidural anaesthesia which alleviated postoperative pain
effectively in their SPS patients. Adequate pre-medication is necessary
to prevent spasms caused by the pain on needle insertion [26,27], although therapeutic medications for SPS such as baclofen and diazepam may be sufficient. Yamamoto et al.
[26]
reported that explaining epidural anaesthesia to the patient
preoperatively reduces fear and anxiety which can induce SPS symptoms.
Shanthanna [27] advocated that using conscious sedation would also keep the patient calm. Elkassabany et al. [23]
demonstrated that somatic paravertebral blockade in their SPS patient,
supplemented with conscious sedation, also prevented postoperative
hypotonia. Spinal anaesthesia is an alternative but Shanthanna suggested
using lower doses to reduce the risk of respiratory distress from a
high spinal anaesthesia, especially in SPS patients who have rigid chest
wall muscles [27].
Great care should be taken when administering spinal or epidural
anaesthesia in SPS patients who have an intrathecal baclofen pump.
Lastly, another route of anaesthesia that has
successfully managed a SPS patient requiring GA was total intravenous
anaesthesia (TIVA). Ledowski and Russell reported that it did not lead
to any postoperative hypotonia or SPS symptoms of muscle rigidity or
spasms [24].
Their patient was undergoing ENT surgery and was administrated propofol
with high-dose opioids which obviated the need for neuromuscular
blocking drugs [24]. Yagan et al. [28]
also followed a similar routine for an orthopaedic procedure without
neuromuscular blocking drugs for intubation and achieved good outcomes.
However, this method may not be applicable in all kinds of surgery,
especially abdominal surgery.
Conclusion
SPS is a rare disease that can present challenges in
anaesthesia due to its effects on the GABA pathway. While minor
procedures can be performed under monitored anaesthetic care with IV
sedation [29],
major cases would require TIVA and RA (with or without conscious
sedation) or a combination of both are possible ways to prevent
postoperative hypotonia or mechanical ventilation but may not be
feasible in all types of surgery. Explaining the procedure and surgery
to the patient with the use of midazolam at induction can keep the
patient calm and relaxed to prevent triggering any SPS symptoms. The use
of propofol at induction with the regular opioids for maintenance of
anaesthesia would be a suitable combination for SPS patients. Volatile
gases may be safe for maintenance too. Rocuronium can also be utilised
especially for difficult intubations with sugammadex at hand if
necessary.
Another recommendation would also be the need for
continuous monitoring of patient parameters such as TOF and BIS which
aid in keeping the doses of neuromuscular blockers and volatile
agents/propofol to a minimum respectively. This is particularly crucial
for patients on long-term baclofen who would also require appropriate
temperature regulation during surgery. Admission to ICU postoperatively
would be pertinent for any major or complicated procedures to check for
any respiratory or muscular complications and to regulate the
readministration of preoperative doses of benzodiazepines.
The data on SPS is limited (only 150 cases from 1980 to 2005 have been described in literature [22]).
Other factors to consider during anaesthesia would be comorbidities and
complexity of surgery performed since it may affect choice of drugs and
route. As all the case reports highlighted in this review involved a
different surgery, more detailed trials would be required to confirm
their findings but the incidence of this disease remains extremely low.
Acknowledgment
The authors acknowledge the valuable editorial input of Professor Ian Mackay.
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php
Comments
Post a Comment