Intranasal Remifentanil as an Adjunct to Oral Midazolam Sedation in Pediatric Dental Patients -Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Purpose: Oral midazolam (MID) sedation is
often used for pediatric dental procedures, however the quality of
procedural sedation is variable and maybe inadequate for successful
procedure completion. Intranasal (IN) sedative administration can be a
useful adjunct as it has a more rapid onset and can be repeated as
needed. MID can also be given IN however it burns and often results in
crying. Routinely we use adjunct IN sufentanil, but due to a prolonged
drug shortage and unavailability (> 1 year) we decided to assess the
efficacy and safety of IN remifentanil (REMI), an ultra short acting
synthetic opiate. There are only a few papers on IN REMI use. One report
of 150 children given IN REMI as an adjunct to intubation did not find
any problems and the kinetics appeared to demonstrate a fast onset of IN
REMI and a rapid elimination as expected.
Methods: We performed at retrospective chart
and QA database review of this novel sedation technique. Children
scheduled for elective moderate dental sedation procedures were given
0.7mg/kg oral midazolam. Since the shortage of Sufentanil we started
using REMI in June 2012.
We gave 2 to 4 doses (2mcg/kg) of IN REMI (Maximum REMI dose used was 40
mcg). The first dose was given in the PREAN area 25 minutes after the
MID dose. The next dose was given when the child was in the dental chair
and monitoring applied in the operatory. Any subsequent doses were
given at a minimum of 5 minute intervals at the request of the dentist
performing the procedure. All patients were monitored with pulse
oximetry, HR and NIBP. The quality of sedation, airway complications and
discharge times were assessed as part of our QA process. The IN REMI
was prepared each day at a 100 mcg/ml concentration (in NS) and
administered using a MAD atomizer device. The volume for each spray was
about 0.3-0.4ml. The right nares were used first and then the alternate
nares were used for any repeat dosing.
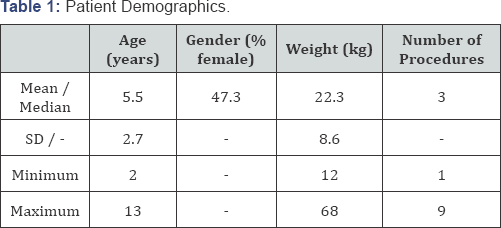
Results: Data was collected on 74 children.
The mean age was 5.5 years (Range 2 - 13) and the mean weight was 22.3kg
(Range 12-68). The mean MID dose was 12.6.mg, 51% were female. The
median number of dental procedures performed on each child was 3 (range
1-9). The mean total dose of REMI given was 94 mcg. The median number of
REMI doses given was 3 (range 2-4). The depth of sedation was assessed
using the RASS score. The median RASS score at: MID dosing, Entry into
the Operatory, During the Procedure, and arrival in the PACU were: 0,
-1, -1, and 1, respectively. The mean procedure duration was 30 minutes.
And the mean discharge time was 50 minutes. The median dental
assessment score was 5.0. All procedures were completely successfully.
There were 2 airway complications noted. Two patients had a desaturation
to 90, this was treated with supplemental oxygen in 1 child (2 REMI
doses) and no treatment was required in the other (3 REMI doses). There
were no episodes of apnea, airway obstruction, or bradycardia.
Conclusion: The REMI appeared to be an
effective adjunct to oral MID. Due to its short half life, repeat dosing
of the REMI appears necessary to obtain an useful duration of effect.
Although we had minimal respiratory side effects, as there is a risk of
apnea and rigidity from this potent opiate we decided to titrate the
dosing of REMI to the desired effect. The optimal dosage strategy still
needs to be determined.
Introduction
Oral sedatives are often required for children who
require dental restoration. Most children are able to receive this in
the dental office, often using nitrous oxide with minimal sedation to
facilitate the procedure. However, there are children who will not
cooperate during the examination process or during the actual dental
procedure without sedation [1].
This can be due to young age, behavior issues or non-compliant
behavior. As a result, these children are scheduled for oral moderate
sedation in the office setting. We routinely use oral midazolam as our
sedation method. Midazolam has a wide safety margin and is effective in
about 80% of the patients [2].
We use a dose of 1 mg/kg up to a maximum of 20mg, wait 30 minutes where
the child is monitored in the preoperative area before going into the
operating room.
In 2012 and 2013, there was a national drug shortage
affecting sedatives and other anesthetic agents. This resulted in
several months of very short supply of oral midazolam. With a waiting
list of over 3 months, cancelling these childrens' procedures would
result in added burden for their families and them. We decided to
evaluate an alternative sedation regimen that would conserve oral
midazolam use. In the past, we have used intranasal sufentanil as an
adjunct to oral midazolam. However during this drug shortage period, the
only parenteral opiate that was available was remifentanil.
Remifentanil is a newer synthetic opiate that has a rapid onset and a
very short half-life of 8 minutes [3,4].
In addition, remifentanil has a very short and stable
context- sensitive half time. As a result, it has been used
intravenously in pediatric patients including critically ill neonates.
It has pronounced cardiac stability and is a potent respiratory
depressant [5].
It usually administered by infusion and IV bolus use has been also
reported but the risks of apnea and muscle rigidity have limited this
approach [6,7]. There appears to be very limited experience with using remifentanil intranasally. A paper by Verghese et al. [8]
published 2008 in Anesthesia and Analgesia, reported the use of
intranasal remifentanil and intubating conditions in a study involving
188 children aged 1 to 7 years. Remifentanil was dosed at 4mcg/kg after
induction of anesthesia, some patients had blood levels checked for
kinetic analysis. Peak plasma levels occurred after 4 minutes and
intubating conditions were superior in the remifentanil group compared
to the placebo. There were no side effects or complications noted
secondary to the remifentanil [8].
We initiated a quality assessment (QA) review process to evaluate this
new adjunct sedation medication. We were interested in the efficacy,
side effects and the effective dose. The aim of this report is to
describe our experience with intranasal remifentanil as an adjunct to
oral midazolam sedation.
Methods
For the QA process, a nurse not involved in the
clinical care of the child collected data prospectively. Data collection
included patient demographics, drug dosing and administration times,
sedation quality, number of dental procedures and complications such as
desaturation, bradycardia, muscle rigidity or tachyphylaxis.
After this QA process was completed and the results
discussed in our department we obtained IRB approval for the publication
of this data from a retrospective review of the QA database. The
sedation method included 0.7mg/kg oral midazolam (maximum dose 14mg)
with the routine ASA monitoring. The first dose of the remifentanil was
given in the preoperative area with the parents present. Remifentanil
solution does not cause pain on administration [9].
Five minutes later the child was taken to the operating room and
monitored with pulse oximetry, heart rate and non-invasive blood
pressure. Oxygen was delivered via nasal cannula. Naloxone and
flumazenil were immediately available for intranasal administration if
required.
The concentration, dose of remifentanil and the
number of administrations evolved as we gathered more experience with
the technique. Initially we used 50 mcg/ml giving 1 or 2 doses of 1
mcg/kg and eventually we used 100 mcg/ml, 2 mcg/kg doseup to 4 doses as
required. The maximum dose used was 60 mcg. The 1mg vial of remifentanil
powder was dissolved in saline to produce the desired concentration.
Then 0.6 ml of remifentanil was then drawn up into 1 ml luer lock
syringes for use during the day, all unused remifentanil was wasted and
documented after the day was complete. All doses were weight based and
drawn into luer lock syringes. All remifentanil was administered using a
disposable mucosal atomization device (MAD®, LMANA). This is a device
that attaches to a luer lock syringe and deposits a fine spray during
administration, ensuring an even spread of the medication onto the
mucosal surface of the nose. Further doses were given as deemed
indicated by the operating dentist.
Sedation quality was assessed using the Richmond
Agitation Sedation Score (RASS). The QA observer assessed this at
various times during the procedure. Also the dentist and the observer
independently rated the overall quality of the sedation on a visual
analogue scale (VAS), 1 - 10.
Results
REMI: Remifentanil
MID: Midazolam
The database review yielded data on 74 patients who
received oral midazolam and intranasal remifentanil. Patient
demographics are shown in Table 1.
The mean patient age was 5.5 years. Each patient had a median of 3
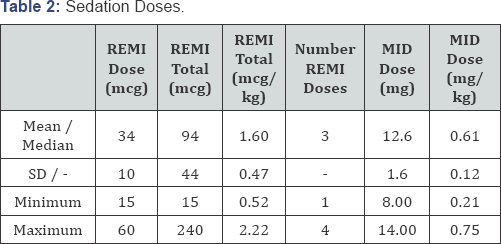
teeth procedures performed during the sedation. The drug doses are shown
in Table 2.
There was a significant variation in the dosing of remifentanil as we
changed our dosing schedule several times. The midazolam dose was within
the dosing parameters of this technique. Remifentanil dosing was
divided into three different schedules (Table 3).
The initial evaluation (low, n= 11), the second dosing schedule
(intermediate, n= 10) and the final administrations. Most of our
experience reported is with the high evaluation (high, n= SS). Dose
escalation occurred with both dose regimen with a mean total
remifentanil dose of 1.8mcg/kg.

REMI: Remifentanil
MID: Midazolam
REMI: Remifentanil
REMI1 to REMI2: time interval between first and second remifentanil dose.
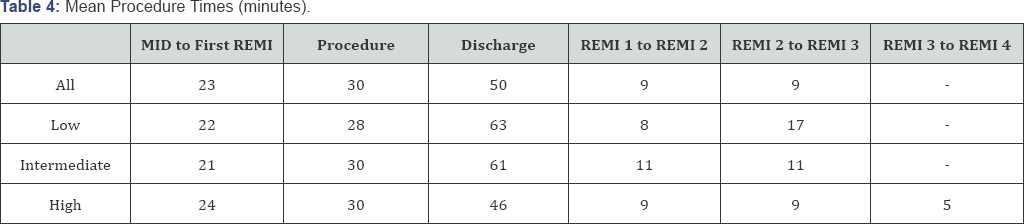
Procedure times are shown in Table 4.
The mean procedure time was 30 minutes and discharge time of 55
minutes. We compared this to data from our sedation QA database for full
dose oral midazolam, (data taken from the six months period prior to
remifentanil use, n=83). The full dose midazolam had a mean discharge
time of 70 minutes, this is significantly longer (p<0.01) than the IN
remifentanil patients. The quality of the sedation appeared to be
significantly better with the high dose group of patients (Table 5).
The RASS scores between the three groups were similar for oral dosing
of midazolam, initial IN remifentanil dose and entering the operator.
The high dose group had significantly better RASS for the procedure
(p=0.004), compared to the other dosing schedules. The benefit was also
noted initially in the recovery room (p=0.02). The dentist's VAS
assessment (Table 6) was significantly better for the high dose group (p=0.004), as was the observer’s VAS assessment (p=0.001).

MID: Midazolam administration
FIRST REMI: First remifentanil dose given
REMI2: Second remifentanil dose give

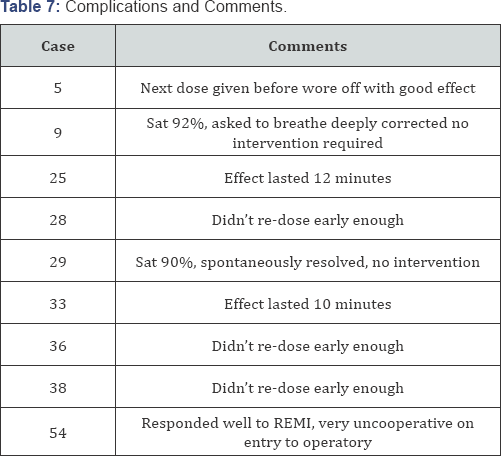
Two patients experienced desaturation episodes (Table 7).
One in the low dose group (Patient 9) and one in the high dose group
(patient 25). There were no cardiac complications. There were no
complaints of nausea or vomiting, chest rigidity or tachyphylaxis.
Comments noted during the QA review included several concerning the
short duration of effect (10-12 minutes) from the IN remifentanil and
the need to re-dose before the effect had worn off. In fact, the next
dose was most effective if given before the child became uncooperative.
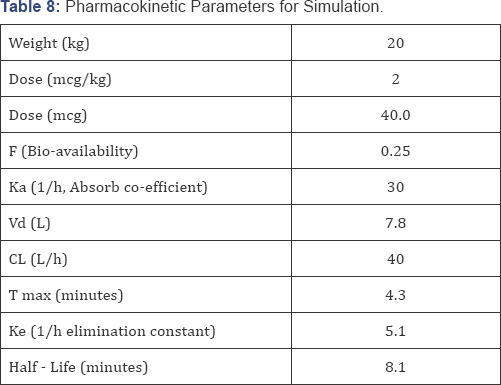
A pharmacokinetic simulation plot (Excel spreadsheet, based upon published kinetic data, Table 8)
shown in Figure i. Demonstrates the changes in remifentanil blood
levels when administering 4 doses of intranasal remifentanil in a manner
as we have reported. The rapid onset of the IN remifentanil allows a
quick increase in the blood level, which also falls quickly due to the
short half-life. The blood level increased with each IN administration,
indicating that a steady state had not yet been achieved .
Discussion
In light of recent drug shortages, this QA report and
subsequent data analysis reviews the off-label use of intranasal
remifentanil for moderate sedation in the pediatric population. In this
study, the higher dose group of 100 mcg/ml with 2 mcg/ kg dose, up to 4
doses provided adequate sedation to these patients with minimal side
effects. In the high dose group the depth of sedation was consistent
with moderate sedation (RASS: -1 or -2) and the procedures were all
completed satisfactorily.
Among all the patients receiving this treatment, only
two episodes of desaturation occurred to 90% with no intervention
required. In addition, no episodes of tachyphylaxis, nausea or chest
rigidity were observed. Intranasal adjunct medication is an attractive
option due to the ability to give multiple doses without requiring the
cooperation of the patient [10-12].
Intranasal medications often have a faster onset, in this case
remifentanil peak effect is within 5 minutes, this facilitates a safe
titration to effect method, as the peak effect can be seen before the
next dose is given. However the intranasal approach reduces the risk of
apnea and rigidity that could be seen with bolus remifentanil, due to
this onset delay [8].
The remifentanil must be diluted into an appropriate concentration, the
optimal volume for an intranasal mediation is less than 0.5 ml, larger
volumes result in a greater degree of unpredictability due to delayed
swallowed medication effect, sensitive to the effects of first pass
metabolism. The volume we used for all cases was a maximum of 0.6 ml,
irrespective of the dose given.
Whenever a drug must be mixed, this increases the
risk for error, also a small volume error of 0.1 ml could result in a
dose error of 25% [13].
When using potent opiates as part of a sedation method, extra care must
be taken with the preparation and administration. A simple reminder,
the volume should never be greater than 0.6 ml could help limit the risk
of a severe overdose.
The use of the MAD improves the distribution of
intranasal administered medications. There is a small dead space volume
(0.05 ml) that should not be a problem unless very small volumes are
being used [14].
The pharmacokinetic simulation demonstrates however,
that this dosing regimen can still result in remifentanil levels that
could cause apnea. Higher blood levels of remifentanil in a patient who
has also received benzodiazepine further increases this potential risk.
The study by Verghese et al. [8]
demonstrated no problems using 4 mcg/kg as a single dose, however in
these patients apnea was not a problem and actually desired, to
facilitate the intubation process, this was not the endpoint in our
analysis. We used a step-wise dosing schedule to evaluate the effects of
remifentanil, increasing the individual dose as well as the number of
doses in a structured manner to ensure that safe sedation was given. The
high dose remifentanil schedule appears to be a safe and effective
dosing method, using 3-4 doses with at least 5 minutes between each
subsequent dose.
Remifentanil is one of the more higher cost sedation
agents available at present. A 1mg vial from our supplier costs about
$70. If this can be used between multiple patients then the cost may be
acceptable. This report has several limitations. It is a retrospective
review of a QA database. There was also no randomization of patients nor
blinding of the observer or dentists. The patients were a convenience
sample that presented to the clinic on days we were able to do the QA
analysis and as such are reflective of our sedation population. However
our population may be significantly different than those in other
university or private offices. Therefore, a single center study may not
be generalizable. This is the first report of the use of intranasal
remifentanil as a sedative in pediatric patients. There may be several
other opportunities for such a rapid acting, titratable, painless non-IV
based parenteral sedation method such as patients requiring short
painful procedures, patients with chronic pain for break-through
management or as an anesthesia premedication.
Conclusion
As a result of the national shortage of drug supply,
this review explored unique modalities for administration of
remifentanil in a pediatric dental population. The intranasal
remifentanil appeared safe and effective, however it was labor intensive
due to the multiple dosing iteration schedule we utilized for safety
reasons. Due to the small sample size, and limited demographic, further
prospective randomized studies are necessary in both the adult and
pediatric populations.
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php
Comments
Post a Comment