Current State of Pharmacodynamic Target Attainment in Critically Ill Patients with Severe Sepsis in Canadian Icus: Prospective Cohort Study-Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Objectives: We sought to
determine the rate of pharmacodynamic target attainment of antibiotic
therapy for critically ill patients with severe sepsis in Canadian ICUs.
Methods: In this multicenter
observational study, adult Canadian ICU patients with severe sepsis were
prospectively followed. The minimum inhibitory concentration (MIC) of
the organism was obtained from hospital’s microbiology laboratory, and
pharmacodynamic targets (PD) of the prescribed empiric antibiotics were
calculated using population pharmacokinetic parameters to estimate the
rate of PD target attainment. The primary outcome was the proportion of
patients who attained PD target. We performed sensitivity analysis
varying the elimination rate constant (ke), volume of distribution (Vd),
and MIC parameters.
Results: Sixty-nine pairs of
antibiotic and MIC were evaluated in 43 patients. In the base case,
PDtarget attainment was achieved in 92.7% (64/69) of the antibiotic-MIC
pairings, or in 88% (38/43) of patients. In sensitivity analysis, 83.7%
(36/43) of the patients achieved the desired PDtarget at all variations
of the PK or MIC parameters. Clinical failure occurred in three patients
despite target attainment.
Conclusions: Current antibiotic
doses for treatment of critically ill patients with severe sepsis
achieved 88% PD target attainment. These results need to be validated
using measured patient PK parameters and a larger sample size of MICs of
causative organisms.
Keywords: Pharmacokinetic; Pharmacodynamic; Critically ill; ICU; Severe sepsisAbbreviations: AUC: Area Under The Curve; ICU: Intensive Care Unit; Ke: Elimination Rate Constant; MIC: Minimum Inhibitory Concentration; PK: Pharmacokinetic; PD: Pharmacodynamic; Vd: Volume of Distribution
Introduction
Optimal use of antibiotics in patients with severe
sepsis is paramount, particularly in this era of increasing antibiotic
resistance, lack of new antibiotic agents being developed, and the high
incidence and mortality associated with severe sepsis [1-3]. In patients
with severe sepsis, optimal antibiotic use involved administering the
right antibiotic in the optimal dose/regimen in a timely manner [4-5].
In other words, therapeutic failures can still occur even if standard
doses of an effective antibiotic are administered promptly. Possible
reasons for this include: alterations in pharmacokinetic
(PK)/pharmacodynamic (PD) parameters in patients with severe sepsis,
compromised organ function as a sequalae of sepsis (e.g. renal and or
hepatic dysfunction), and inadequate local antibiotic concentration
(e.g. abscess) despite adequate serum concentrations. Therefore,
alternative dosing strategies that incorporate both PK parameters of the
patient and PD properties of the antibiotic
have been advocated, such as the use of continuous or extended
interval infusions, particularly for critically ill patients with
severe sepsis [6]. This is consistent with the recommendations
set out by the Infectious Diseases Society of America, in which
dosage optimization is suggested as a possible strategy for
improving the usage of current antibiotics and minimizing the
development of resistant organisms [7].
Patients with severe sepsis represent a highly vulnerable
population with significant risk of morbidity and mortality
who might benefit from dosage optimization efforts. However,
the extent to which current doses of antibiotic regimens for
patients with severe sepsis achieve optimal PD targets is
unknown. Literature surrounding this issue consists primarily
of non-clinical studies demonstrating theoretical benefits, nonrandomized
or controlled clinical studies with positive results.
Furthermore, the use of extended/continuous infusion in an
attempt to optimize the probability of PD target attainment also
yielded conflicting results as reported in a recent systematic
review of randomized controlled trials [6]. The objective of this
study is to determine the rate of attainment of pre-defined PD
targets for critically ill patients with severe sepsis.
Materials and Methods
Study design, setting and patient population
This multicenter, prospective, observational trial enrolled
patients admitted to an ICU between September 2012 and June
of 2013. Patients ≥ 18 years old with severe sepsis were eligible
for enrollment into the study. Severe sepsis is defined using
the standard consensus criteria [8]. Patients were excluded if
they were: 1) Immunocompromised, defined as: those with an
absolute neutropenia count of less than 500/mm3, patients
with active human immunodeficiency virus infection and
not on highly active antiretroviral therapy, patients receiving
greater than 20mg of prednisone equivalents per day for greater
than one month, patients on concurrent chemotherapy, or on
immunosuppressive therapy post organ transplantation, 2) on
antibiotics for less than 24 hours, and 3) receiving only antifungal
or antiviral medication. Immunocompromised patients were
excluded as the management of their infections often require
different approaches and thus limit generalizability.
Data collection
For each participating ICU, all patients were screened daily
and enrolled patients were followed starting from the initiation
of empiric antibiotics for the treatment of their severe sepsis for
14 days, or until discharge from the ICU or death/withdrawal of
care, whichever occurred first. Data was collected prospectively
only for the first episode of infection for each patient regardless
of when it occurred during their ICU stay, or if the patient was
readmitted to the ICU. A standardized case report form was
used to ensure all relevant information was collected for the index episode of infection including microbiology results.
The MIC of the causative organism was determined by the
participating hospital’s microbiology laboratory using current
local techniques. Causative organism was defined as the isolate
identified from the presumed infectious source as documented
in the medical records. No attempts were made to alter current
practices, including antibiotic dosing which were determined by
local care team and in accordance with product monographs of
each drug. Dosage adjustment for patients with renal dysfunction
was determined by local care team as well. Given the multicenter
nature of the study, standard definitions were developed for
each data point and were made available to all participating
sites prior to the beginning of patient enrollment. Clinical
cure, reported by each site investigator without independent
adjudication, was defined as complete resolution of the signs/
symptoms associated with the infection and discontinuation of
the prescribed antibiotics. For patients receiving combination
antibiotics, either as initial empiric or definitive therapy, each
antibiotic - MIC pairing was evaluated separately.
Pharmacokinetic data used for calculations
A comprehensive review of all published literature was
performed for each antibiotic reported by the sites using
MEDLINE (1946-2012) using the keywords “drug monitoring”,
“pharmacokinetics”, “sepsis”, “critical care”, and “critical illness”.
Articles were restricted to those written in English and included
patients aged 18 years or older. All abstracts recovered were
reviewed by one investigator (J.P.) using the following criteria:
[1] describing the use of the antibiotic in question in patients
admitted to the ICU or in patients with severe sepsis, and
[2] providing pharmacokinetic data of the antibiotic in the
population of interest. The hierarchy of choice of PK parameters
from the literature, in descending order, is: ICU patients with
severe sepsis, general ICU patients, hospitalized patients, and
healthy volunteers. The references of included manuscripts were
searched for other potential manuscripts that might qualify for
inclusion. For qualifying abstracts, full text manuscripts were
reviewed by the same investigator (J.P.) for the following data
elements: (1) volume of distribution (Vd), and (2) the estimated
elimination rate constant (ke). In situations where the Vd was
reported in units of liters per kilogram (L/kg), an assumption
of 70 kilograms was made. In some published literature, half life
(t1/2) was reported instead of ke, and the ke was calculated using
the following equation (ke = ln2/t1/2). The un weighted mean
values of the Vd and ke for each antibiotic was calculated if there
existed more than one study. If values are available for patients
on renal replacement therapies (intermittent or continuous
modalities) these were also abstracted. These PK parameters
were used for calculations in the current study.
Pharmacodynamic target attainment calculations
The primary outcome of this study is the proportion of
infections treated with an antibiotic regimen that is predicted to achieve the desired PD targets derived from current
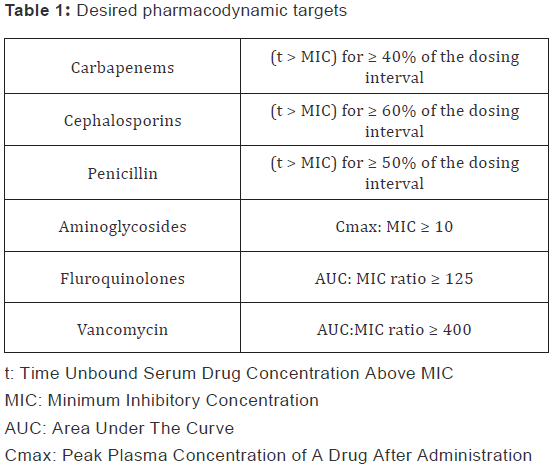
literature (Table 1) [9,10]. Target attainment was calculated
by using PK parameters described above, a one-compartment
model, and patient weight. Pharmacodynamic calculations
were performed for each individual patient and the antibiotic
(empiric or targeted) - MIC pairs. Area-under-the-curve (AUC)
was estimated from daily antibiotic dose and patient’s clearance
of that antibiotic. For antibiotics that exhibit time dependent
killing, time above MIC (t > MIC) was estimated using one
compartment kinetic modeling. These calculations yielded the
base case scenario used to determine the primary endpoint.
Given the inherent variability in PK parameters (see table 1) and
in automated methods of MIC determination commonly used at
the participating institutions, we conducted sensitivity analysis
of the base case scenarios using the following variables and
range: 1) one fold increase/decrease in MIC values (common
range of error for the automated methods), 2) minimum and
maximum literature reported values in PK parameters of
clearance and volume of distribution.
The study protocol and waiver of consent was approved by
the institutional review boards at each of the participating sites.
Results
Participating sites demographics
Seven Canadian ICUs participated in this study. The ICUs were
all in teaching institutions, predominantly closed ICU (6/7), and
cared for a mixed population (medical, surgical, and some trauma
and neurosurgical). Antimicrobial stewardship programs were
present in 4/7 ICUs, and standard sepsis management protocols
were present in only 2 ICUs. All but one institution used the
VITEK system for automated MIC determination, with Micro
scan being used in the remaining ICU. However, only 2/7 ICUs
routinely receive the MIC values with the microbiology report.
All the ICUs have dedicated clinical pharmacist (s) assigned.
Episodes of inpatient care
Of the patients included, 360 (57%) had at least one episode
of inpatient care within the 3 years before the admission to ICU.
The ICU patients with pre-existing diseases used significantly
more hospital resources 3, 2, and 1 year before admission to
the ICU (p= 0.008, <0.001, and <0.001), and up to 3 years after
discharge regarding amount of care (number of visits) (p=
0.003), duration of stay (p=0.001), and costs (p=0.002), but
there were no differences during the ICU period in that preexisting
disease did not increase the cost of the stay in ICU. Most
importantly, there was a significant increase in the number of
episodes of inpatient care for the 3 years before admission to
ICU (p<0.001).
Patient characteristic
Forty-three patients (30 males and 13 females) were enrolled
in the study (Table 2). These patients were moderately ill with a
mean APACHE II score of 22 ± 6.5. At baseline, the most common
site of infection was blood (24 patients), and the most common
organ system failure was cardiovascular. A variety of causative
organisms were reported, with E. coli being the most common
gram-negative organism and methicillin-sensitive S. aureus
being the most common gram-positive organism. Seventy eight
percent of patients had 3 or more SIRS criteria, and 28% were
on renal replacement therapy. Dosages of antibiotics prescribed
are outlined in Table 3.
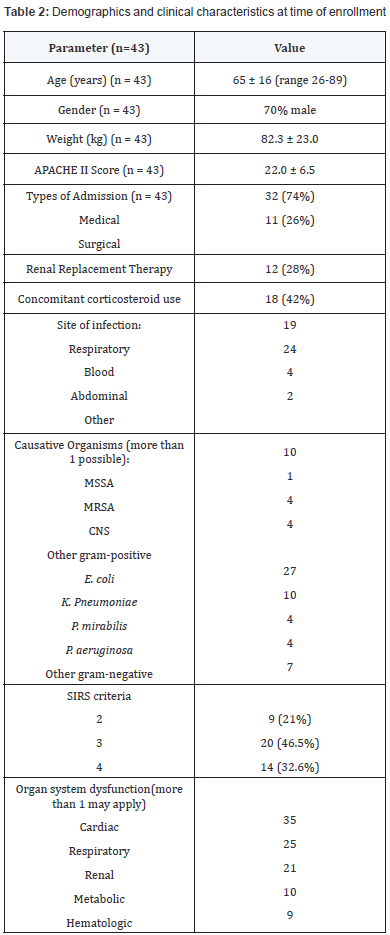 APACHE: Acute physiologic and chronic health adjustment evaluation;
SIRS: Systemic Inflammatory response syndrome; MSSA: Methicillin-
Sensitive S. Aureus; MRSA: Methicilin-Resistant S. Aureus; CNS:
Coagulase-Negative Staphylococci
APACHE: Acute physiologic and chronic health adjustment evaluation;
SIRS: Systemic Inflammatory response syndrome; MSSA: Methicillin-
Sensitive S. Aureus; MRSA: Methicilin-Resistant S. Aureus; CNS:
Coagulase-Negative Staphylococci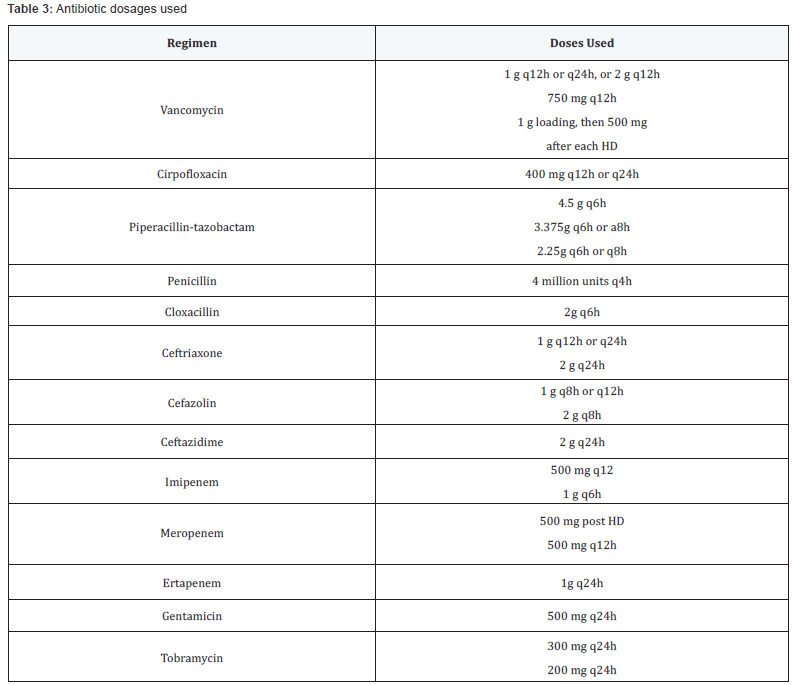 Q24h: Every 24 hours; HD: Hemodialysis
Q24h: Every 24 hours; HD: HemodialysisOutcome analysis
Analysis of 69 pairs of antibiotic and MIC were completed
in all 43 patients. Pharmacodynamic target attainment was achieved in 91% (63/69) of the antibiotic-MIC pairs, or in 88%
(38/43) of the patients in the base case scenario (Table 4).
Sensitivity analysis showed that 84% (36/43) of the patients
achieved the desired pharmacodynamic targets at all variations
of the factors associated with therapeutic failure (one fold
increase and decrease in MIC, minimum and maximum ke and
Vd). Exploring the 5 patients who did not attain the desired PD
targets in the base case scenario, 2 patients were prescribed
antibiotics that the organism has intrinsic resistance towards,
and 3 of the 5 patients were due to a prescribed conservative
tobramycin dosage, but they were also receiving concomitant
antibiotics that did attain PD targets. In the sensitivity analysis,
2 more patients failed to attain PD targets when the MIC
values were doubled. Three patients were classified as clinical
failures, and in all cases PD targets were achieved (Table 5). One
patient with clinical failure had concomitant fungemia, one had
inadequate source control.
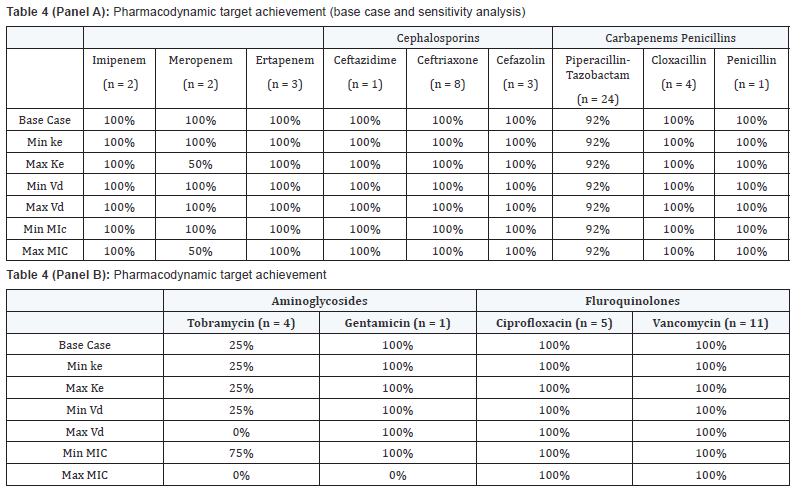 Base case refers to target attainment for average Ke and Vd and
laboratory reported MIC. Ke and Vd were varied using min/max values
found
in literature and MIC were varied with one fold dilution above and below
reported MIC
Base case refers to target attainment for average Ke and Vd and
laboratory reported MIC. Ke and Vd were varied using min/max values
found
in literature and MIC were varied with one fold dilution above and below
reported MIC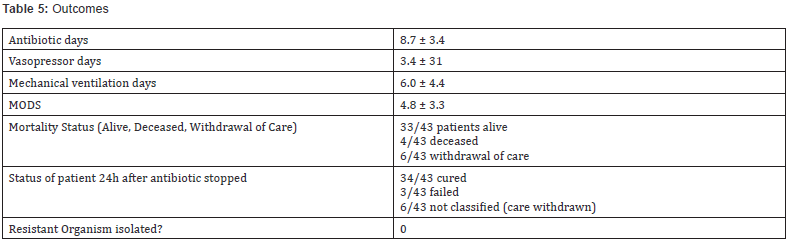 Values are mean ± standard deviation
Values are mean ± standard deviationMODS: Multiple organ dysfunction score
Discussion
In this prospective, observational, multicenter study, using
clinical data from 43 critically ill patients with severe sepsis
treated with antibiotics, we found that 88% of patients achieved
PD targets. In the sensitivity analysis, 84% of patients achieved
the desired PD targets at all variations of the Vd, ke and MIC.
To the authors’ knowledge, this is the first multi-center
study documenting the proportion of ICU patients with severe
sepsis achieving pre-defined PD targets with a variety of
antibiotic therapies. The results garnered from this study
differs significantly from a single center study that enrolled 19
ICU patients with gram negative sepsis that reported only 16%
(3/19) of the patients achieved the desired PD targets [11]. The
differences may be explained by the different PD targets used
during that study timeframe, smaller sample size, and different
microbiologic ecology of the study sites. The recently published
DALI study involving 181 culture-positive ICU patients with any
infections (out of the total 361 patients included in the study)
reported that 16% of patients did not achieve the defined PD
target, similar to our findings despite different methodology
[12]. The DALI study enrolled a different patient population (severe sepsis vs any infection), evaluated different antibiotics
(beta-lactam only), and partially used measured MIC (only 34.2%
of the pathogens had an MIC value, and the remaining cases were
analyzed using the literature reported MIC90). The DALI study
also performed actual PK parameter determination, and used PD
targets which were also different (total drug concentration above
MIC for 40-60% of dosing interval vs free drug concentration
above MIC for 50% of dosing interval). While a 12-16% target
non-attainment rate is undesirable from a patient perspective,
both the DALI and this study have limitations that preclude
precise estimates of the true target non-attainment rates. In
our study, target non-attainment did not predict clinical failure,
while in the DALI study target non-attainment was found to be
a significant risk factor for poor clinical outcomes. Therefore
efforts to maximize probability of target attainment may be
more appropriately reserved for at risk patient population or
organisms with known borderline MIC values, or in patients
with PK parameters that would predict failure. This differential
importance is consistent with the conflicting literature that
demonstrate any clinical benefits of using strategies such
as extended/continuous infusions of antibiotics in order to
optimize PD target attainment [6,13,14]. While the principles
underlying PD based dosing is intuitive and supported by in vitro
studies and small case series, it has not been consistently proven
in larger randomized controlled trials. Closer examination of
these principles and their operationalization in actual clinical
practice provides explanation for this discordance. First, the
desired PD targets published in the literature are usually
derived from single studies with minimal validation by other
investigators. Pharmacokinetics data for these antibiotics
in critically ill patients is not routinely published, and thus
extrapolation from studies in other patient population are often
done, which carries potential for significant errors given the
known variability in the critically ill population. In addition, MIC
values are an important determinant of PD target attainment;
given the inaccuracies associated with MIC values determined
by automated techniques routinely employed in most hospitals,
ascertaining actual PD attainment would only be possible with a
significant increase in workload in the microbiology laboratory
to perform more accurate MIC determinations. Finally, it is clear
even from mathematical calculations that with low MIC values PD
target attainment is easily achieved even at conventional doses
of antibiotics. Therefore before widespread changes in both
microbiology practice and antibiotic dosages are warranted,
more rigorous proof-of-concept of PD-based dosing is required.
Strengths of our study include gathering data from multiple
ICUs across Canada, providing a broader representation of
ICU patients, causative organisms and infectious sources.
Standardized data definitions also ensured data accuracy. The
Canadian context is important given the different bacterial
resistance patterns in different countries especially in comparison
to the US. Sensitivity analysis was performed to enhance internal and external validity. Some important limitations of our study
are inherent in its design: the naturalistic, observational design
precludes proving causality. In addition, the majority of patients
received combination antimicrobial therapy. The use of the
automated VITEK system to estimate MICs instead of the E-test
or traditional Kirby Bauer techniques introduces another layer
of error [15]. Use of population pharmacokinetic parameters
to estimate PD-attainment may not be representative of every
patient enrolled in our study and could not account for any
augmented renal clearance, in addition to lack of documented
PK values in critically ill patients for some antibiotics. However
this is a systemic issue with antibiotic PK in ICU patients in
general and not specific to this study. Our study focused solely
on conventional administration of antibiotics and did not take
into account strategies for optimizing PD targets such as use of
continuous infusions, or use of higher than recommended doses
to achieve PD targets. Finally this study was designed to test the
hypothesis of target attainment, not clinical outcomes, which
would require a much larger sample size.
This study demonstrated a reasonable level of PD target
attainment with current antibiotic doses in Canadian ICU
settings. Studies designed to produce more precise estimates of
this target attainment rate are needed to delineate which clinical
scenario may warrant the efforts to optimize the probability of
PD target attainment.
Acknowledgements
We would like to acknowledge Curtis Harder for assistance
with data collection. We would also like to acknowledge all
the participating sites: St. Michael’s Hospital, Toronto; Mount
Sinai Hospital, Toronto; The Ottawa Hospital, General Campus,
Ottawa; London Health Sciences Center, London; Hôpital du
Sacré-Coeur de Montreal, Montreal; and Eastern Health Hospital,
St. John’s Newfoundland.
Funding
Provided by Medbuy Research and Education funds.
Ethical Approval
All sites received approval from their respective REB.
Authors Contribution
- CC contributed to the design of the study, data collection, data analysis, and wrote the initial draft of the manuscripts and revised subsequent drafts.
- JP and KP contributed to data collection and analysis, and contributed to the draft of manuscripts.
- LB, LK, AJF, SK contributed to study design, data collection and also to the revisions of the manuscripts.
- All authors have approved the final draft of the manuscript.
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php

Comments
Post a Comment