Management of Intrathecal Pump Site Infection in a Patient with Metastatic Breast Cancer without the Removal of the System, a Case Report-Juniper Publishers
Juniper Publishers-Journal of Anesthesia
Abstract
Background: Intrathecal delivery of pain
medication with an implantable infusion pump is being increasingly used
for management of intractable pain in cancer patients. Infection of an
intrathecal pump system is a rare but dangerous complication and usually
leads to the removal of the whole system.
Case-Presentation: The author presents the
case of a 69 year old woman who was diagnosed with metastatic breast
cancer at presentation in 2009. She was treated systematically with
chemotherapy and radiotherapy and underwent intrathecal pump
implantation for targeted opioid delivery. Seven months after the
implant, and a in a location of previous radiotherapy, she developed an
infection at the site of the catheter insertion with exposure of the
catheter itself. Clinically she presented with signs of local infection,
high white blood cell count and back pain.She was treated with
extensive surgical debridement of her back wound, removal the exposed
intrathecal pump catheter and was started on intravenous ertapenem and
later transitioned to levofloxacin and rifampicin for the duration of 3
months.
Conclusion: The author reports a case of
infection following implantation of intrathecal pump in a cancer patient
can be treated with intravenous antibiotics without the removal of the
intrathecal pump system.
Keywords: Methecillin sensitive staphylococcus aureus; Surgical site infection; Bacteroides stercoris, Peptostreptococcus prevotiiAbbreviations: CNS: Central Nervous System; MSSA: Methecillin Sensitive Staphylococcus Aureus; SSI: Surgical Site Infection
Introduction
Intrathecal pump system implies that the medication
is administered directly into the central nervous system, which
potentially reduces the side effects. Previous studies comparing the
efficacy of intrathecal pump in association with medical management
alone showed that at 4 weeks follow-up, 84.5% of patients in the
intrathecal treatment group had clinical success vs. 70.8% in the
conventional medical management group. The follow-up study revealed
significant improvement in 6 months survival and also reductions in
fatigue. [1,2]
There are numerous possible complications documented
after implantation of intrathecal pumps including: post puncture
headache, dislocation or leakage of the catheter, complication regarding
medication inside the pump and infection of the system. The data on the
incidence and management of infections of intrathecal delivery system
in cancer patient population is rather sparse. In analogy with the
protocols for the management of infections of cardiovascular devices,
intrathecal pump system infections can be classified as: pocket site
infections including soft tissue infection around the device and the
segment of the leads (i.e., not the transvenous segment), deeper
infections that are associated with hematogenous spread and spinal cord
infections [3].
Other types of classification include: Primary site
infection, in which the pump and its pocket is the site of infection,
and secondary infection due to bacteremia from a different source.
Intrathecal pump system infection diagnosis is
confirmed by identification or culture of microorganisms (mostly
bacteria) on sample from a suspect surgical site or implant area.
Clinical signs of soft tissue infection can include local tenderness,
fever, erythema, wound exudate, wound dehiscence and or skin erosion at
the site. A rare but potentially fatal complication of infection is the
spread of infection to intrathecal space [4].
Case Description
This is a 69 year old female who had an intrathecal
pain pump placed for treatment of intractable pain secondary to
metastatic breast cancer to the spine, lungs and liver who subsequently
underwent palliative chemotherapy and radiation therapy, including
radiation to the lumbar spine several years prior to presentation. She
presented to the hospital after two months of worsening lumbar area skin
infection which was unsuccessfully treated with local debridement and
was being followed by infectious disease and wound care. Due to
worsening pain and need of a refill of the intrathecal pump, she
presented to the pain clinic. At that time, the infection had worsened
and a portion of the intrathecal catheter was exposed (Figure 1).
She also had some serous fluid discharge from the lumbar wound.
Clinical examination revealed she was oriented and alert and had no
symptoms or signs of central nervous system (CNS) infection or systemic
opioid toxicity.
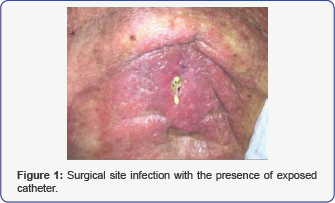
Physical exam revealed the patient to be afebrile and
a lumbar back wound with exposed intrathecal pump catheter and a large
area of cellulitis surrounding it. There was clear involvement of the
spinous processes and the catheter tubing itself. Her blood count was:
Hct=35%, WBC=12.35, (polymorpho nuclear 90%, lymphocytes 7.1%),
Platelets =300,000. Cultures from the site taken during debridement
showed Bacteroides stercoris, Peptostreptococcus prevotii and
methecillin sensitive Staphylococcus aureus (MSSA). Spine CT Scan showed
mild edema within the bones particularly the L2 spinous process and
soft tissue edema. No epidural collection or cauda equina compression
was noted.
Plastic surgery, infectious disease and neurosurgery
were consulted. After informed consent was obtained, she was taken to
the operating room under general anesthesia for extensive wound
debridement, however, upon attempted removal of the catheter, the
catheter was severed and a portion it was left intrathecally. The pump
itself did not appear to be involved and it was left in place. She was
started on intravenous Ertapenem for 8 weeks and switched to
Levofloxacin and Rifampicin. The wound was treated with wound vac and
left open for secondary closure. She was discharged to a rehabilitation
facility in stable condition while her intrathecal pump was infusing
subcutaneously. Her pain was under control with the addition of minor
oral opioids.
Discussion
Significant advances were made in the management of
cancer- related pain with the release of the "pain ladder" by the WHO in
1986, with an update in 1996 [5].
Using this ladder, most cancer patients quickly advance to the use of
strong opioid. As such, opioids continue to be the mainstay treatment
for cancer pain. Unfortunately, when the WHO pain ladder is used,
effective pain control is not achieved in approximately one third of
patients. In addition, approximately 14% of patients do not achieve
effective pain relief at any time [6,7]. For this reason, many pain practitioners have incorporated placing an intrathecal pump in the treatment of cancer pain.
Smith et al. [1]
in 2001 conducted a randomized trial study comparing comprehensive
medical management to medical management plus an intrathecal pump
system. After that study in a subsequent paper with extended follow-up
of the same patients was published in 2005 [1].
These studies found that medical management plus an intrathecal pump
system produced statistically significant reductions in reported pain
scores and opioid related toxicities.
Intrathecal pumps are implanted to treat different
types of pain in cancer patients. Since the intrathecal pump system
releases prescribed amounts of pain medication directly to spine
receptors, pain symptoms can be controlled using a smaller amount
compared to the oral dose. Most people also experience fewer or more
tolerable side effects, such as nausea and constipation, which can be
devastating in cancer patients [8-10].
The major complications of the intrathecal drug
delivery systems after pump malfunction and catheter issues are
infection. The most common post-operative infection for these devices is
a surgical site infection (SSI) [11].
In the context of implantable devices, SSIs are defined as infections
that occur within one year after implantation if the device is not
manipulated and if the infection appears to be related to the operation.
Even though intrathecal pump infection is not as common as other CNS
infection due to prosthetic devices (like shunts), it remains among the
most disabling and feared complications and is of particular concern
because of the threat it poses to CNS infection. The range of infectious
complications in previous reports with intrathecal drug delivery
systems is from 2% to 8% [12].
Risk of surgical site infection in other clean
surgical procedures like breast surgery is elevated in cancer patients
(3-15%) compared to non-cancer patients (3%) [13]. Similarly, we expect a higher risk of infection following intrathecal drug delivery implantation in cancer patients.
General risk factors for SSIs that are pertinent to
cancer patients include leucopenia associated with the cancer or cancer
therapy, diabetes mellitus, debilitated status, poor nutritional status,
smoking and possibly corticosteroid use [14-20].
In addition, cancer patients frequently undergo treatment with
chemotherapeutic agents and radiation, both of which can delay wound
healing, increasing the risk of infection [16].
Infections of Intrathecal pump are dominated by
bacteria of low virulence. The bacteria that are most frequently
cultured in infections are the coagulate negative Staphylococci and
Staphylococcus epidermidis (50-75% cases). Some of these bacteria have a
unique property that appears to facilitate their colonization of
tissue. The treatment of choice is usually considered to be removal of
the entire infected pump system including the catheter and systemic
antibiotics administration.
Conclusion
To our knowledge this was the first report of
treatment of intrathecal drug delivery infection in a cancer patient
without removal of an implanted intrathecal pump. We strongly believe
that in cancer patients with low grade infection and mild clinical
symptoms of infection, removal of intrathecal pump should not be
considered as the first treatment option. We suggest initially starting
conservative treatment including wound debridement and systemic
antibiotics. If the treatment is started promptly, supported by our
case, this may lead to avoidance of at least one surgical procedure and
reduction of patient cost and risk.
For more articles in Journal of Anesthesia
& Intensive Care Medicine please click on:
https://juniperpublishers.com/jaicm/index.php
https://juniperpublishers.com/jaicm/index.php
Comments
Post a Comment